
VENOFER IV 5 ML 5 AMPUL Ravi Specialities Pharma Pvt Ltd., Tiruchirappalli, Tamil Nadu
See full prescribing information for. VENOFER. Venofer is an iron replacement product indicated for the treatment of iron deficiency anemia in patients with chronic kidney disease (CKD). (1) Injection: 50 mg/2.5 mL, 100 mg/5 mL, or 200 mg/10 mL (20 mg/mL) in single-dose vials. (3) Known hypersensitivity to Venofer.

Dónde comprar 100 mg/5 ml solución inyectable
Venofer (iron sucrose injection, USP), an iron replacement product, is a brown, sterile, aqueous, complex of polynuclear iron (III)-hydroxide in sucrose for intravenous use. Iron sucrose injection has a molecular weight of approximately 34,000 to 60,000 daltons and a proposed structural formula: [Na 2 Fe 5 O 8 (OH) ·3(H 2 O)] n ·m(C 12 H 22 O 11)
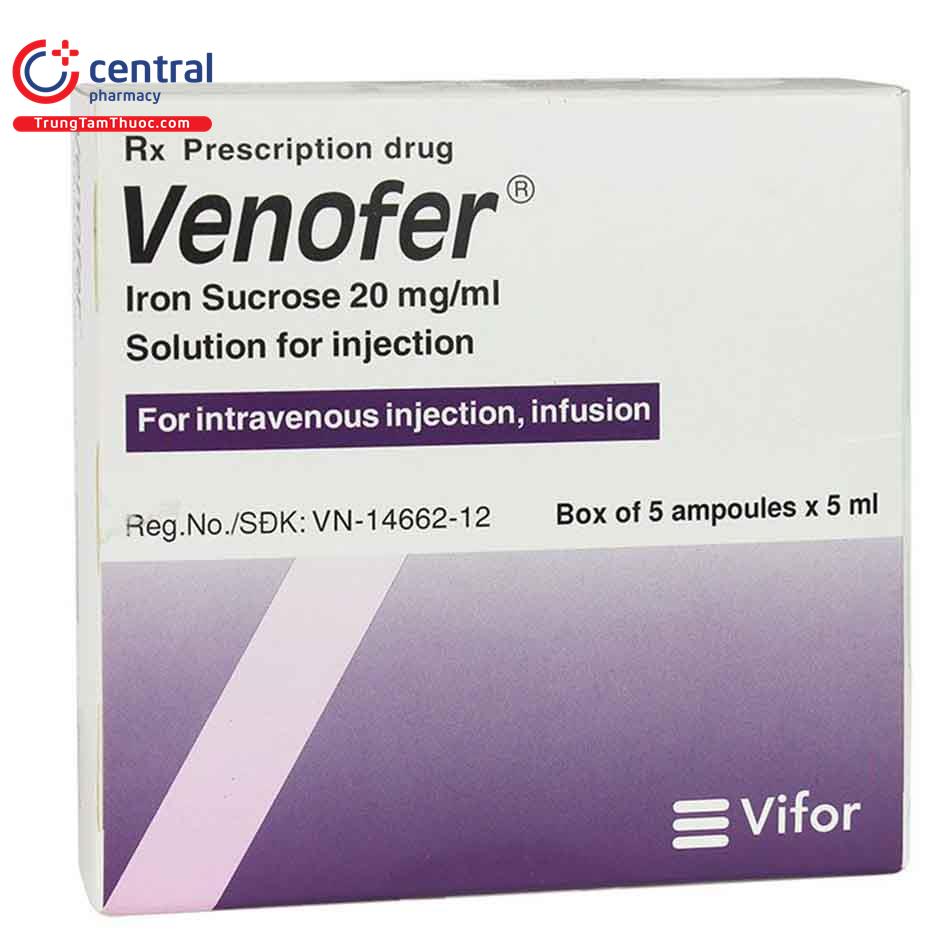
Thuốc truyền tĩnh mạch Venofer 20mg/ml ( lọ 5ml ) điều trị thiếu sắt
Iron sucrose, the active ingredient of Venofer, is composed of a polynuclear iron(III)-hydroxide core surrounded by a large number of non-covalently bound sucrose molecules. The complex has a weight average molecular weight (Mw) of approximately 43 kDa. The polynuclear iron core has a structure similar to that of the core of the physiological.

Venofer 100 mg x 5 ampollas
Venofer ® (iron sucrose) injection, USP is an established and effective treatment for chronic kidney disease (CKD) patients experiencing iron deficiency anemia (IDA). Venofer provides IV iron therapy for the treatment of IDA in adult and pediatric patients 2 years and older with CKD. The dosing for iron replacement treatment in pediatric.
Venofer Injektionslösung 100mg/5ml 5 Ampullen 5ml in der Adler Apotheke
Venofer: Iron sucrose belongs to the class of medications called hematinics . It is used alone or with an erythropoietin (medication that increases red blood cell production) to treat iron deficiency anemia for people who have chronic kidney disease. It may be given regardless of whether you are receiving dialysis or not.
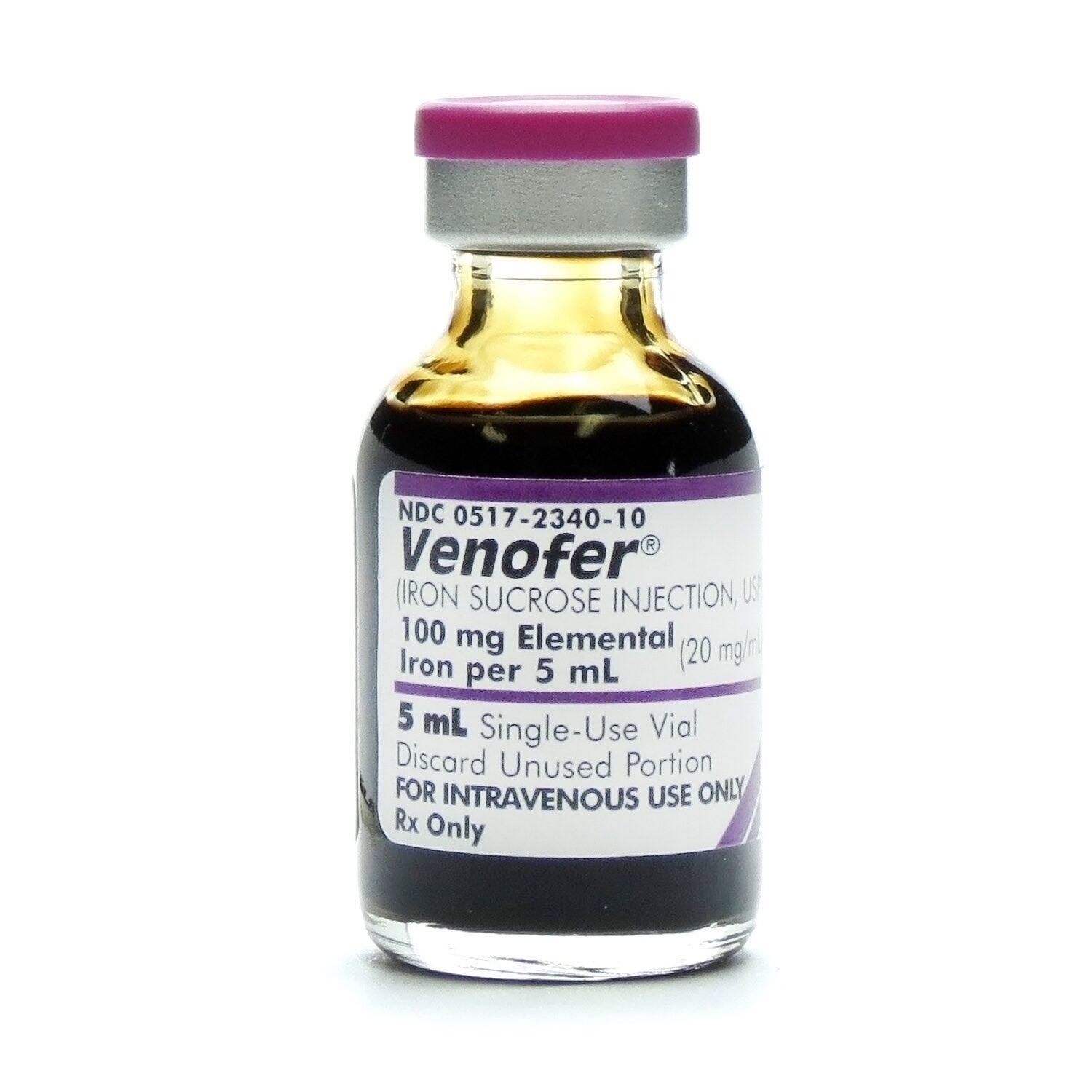
Venofer® (Iron Sucrose Injection, USP), 20mg/mL, SDV, 5mL/Vial McGuff Medical Products
Venofer ® is indicated to treat iron deficiency anemia (IDA) in adult and pediatric patients (2 years and older) with chronic kidney disease (CKD). Venofer ® (iron sucrose) injection, USP is an intravenous (IV) form of iron, which is a key ingredient for making new red blood cells. Iron is also necessary for your bone marrow to build healthy new red blood cells and provide oxygen to your.

Venofer 20 mg Fe/ml 5X5 ml
VENOFER. Venofer is an iron replacement product indicated for the treatment of iron deficiency anemia (IDA) in patients with chronic kidney disease (CKD). (1) Injection: 50 mg/2.5 mL, 100 mg/5 mL, or 200 mg/10 mL (20 mg/mL) in single-dose vials. (3) Known hypersensitivity to Venofer.
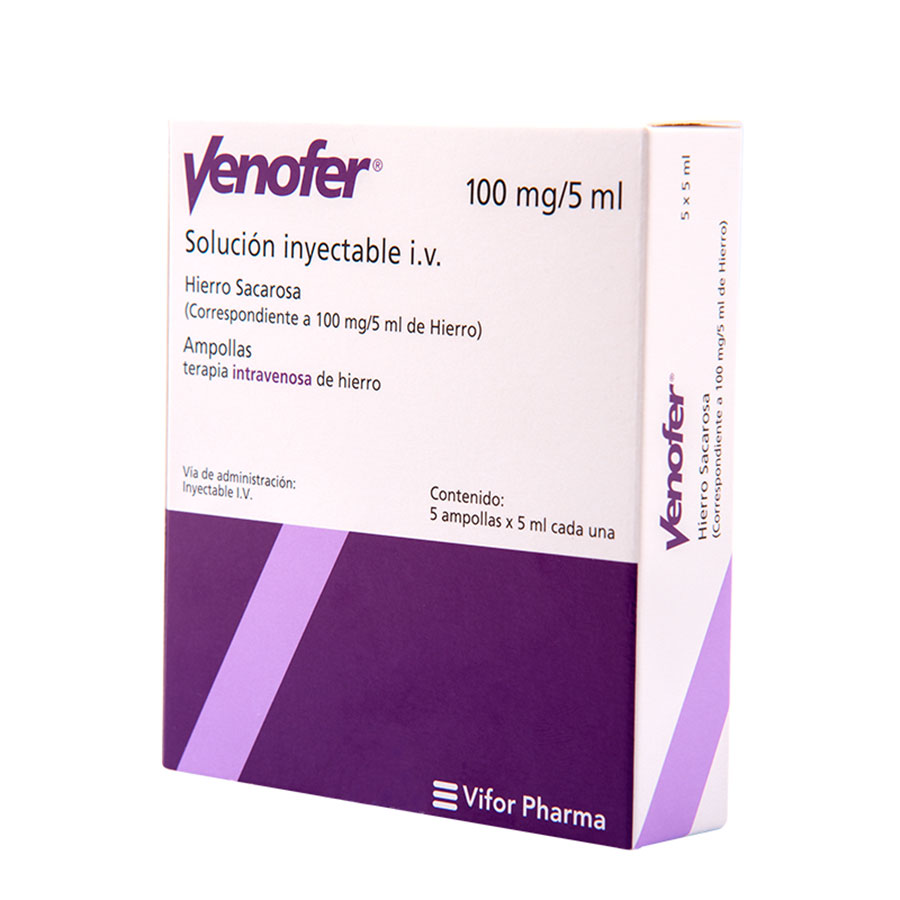
VENOFER x 5 Solución Inyectable
VENOFER. Venofer is an iron replacement product indicated for the treatment of iron deficiency anemia (IDA) in patients with chronic kidney disease (CKD). (1) Injection: 50 mg/2.5 mL, 100 mg/5 mL, or 200 mg/10 mL (20 mg/mL) in single-dose vials. (3) Known hypersensitivity to Venofer.
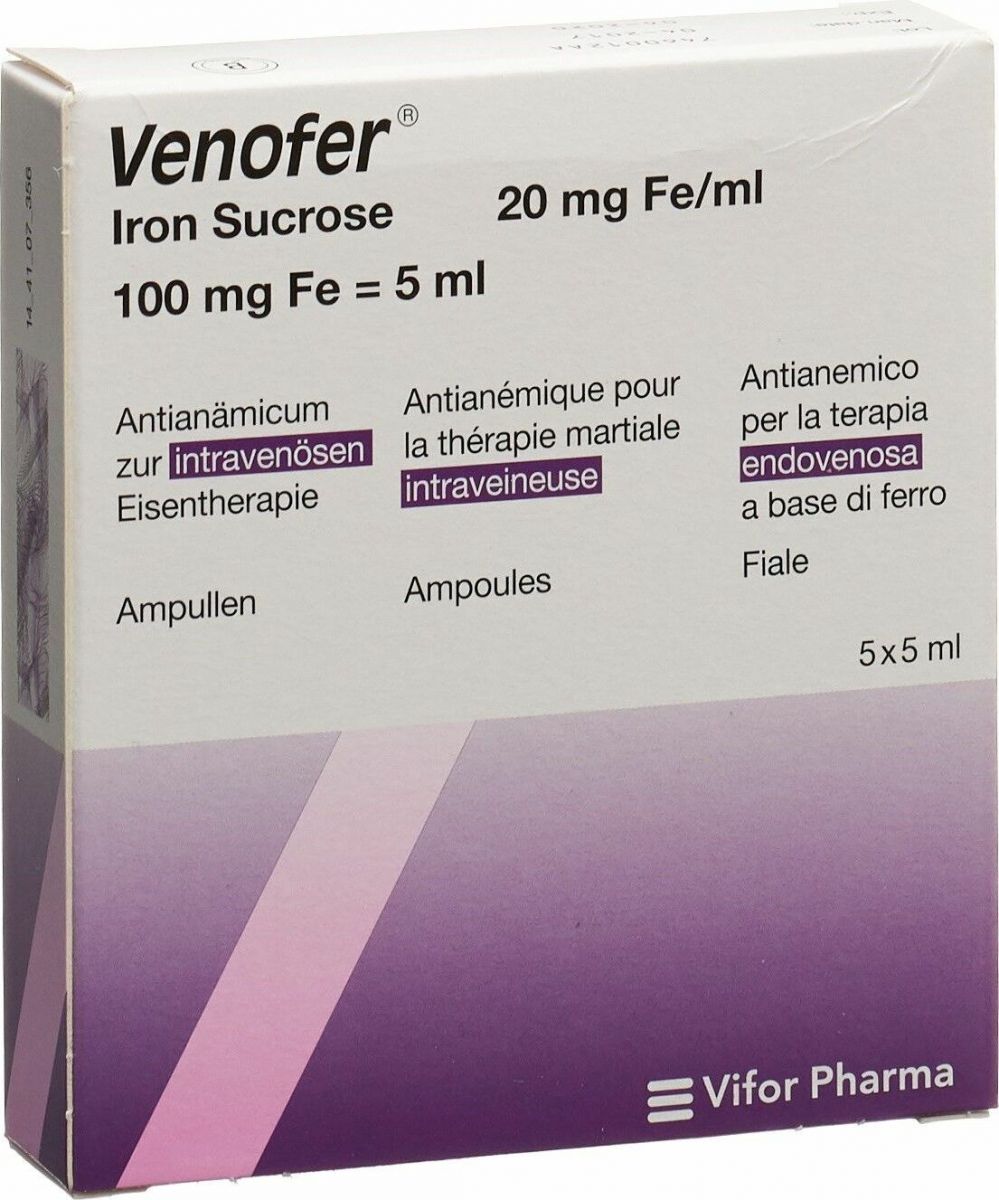
Venofer 100 mg Fe/5 ml 5x5 ml mit dem ERezept kaufen SHOP APOTHEKE
difficult or labored breathing. dizziness, faintness, or lightheadedness when getting up suddenly from a lying or sitting position. headache. nervousness. pounding in the ears. rapid weight gain. slow or fast heartbeat. sweating. tingling of the hands or feet.
Venofer 100 mq 5 ml N5 onlayn aptek
Venofer® (iron sucrose) is a liquid form of the mineral, iron. It is a brown-coloured solution mixed with saline. We give Venofer® through your vein (intravenous or IV). It works quicker than iron taken by mouth. It also does not irritate your stomach or cause constipation. Why do I need Venofer®? We often treat patients with Venofer® if they
VENOFER 20MG/ML OPL INF EN INJ 5ML AMP 5 Apotheek Puttemans
Patients received 200 mg by of Venofer® by slow IV push every week. Analysis showed that 35 out of 42 patients (83.3%) received 4 doses. The mean increase in hemoglobin level after 4 doses was 1.65±0.98 g/dl (P=0.00002). 7 out of 35 patients achieved increase of 2 g/dl or greater in hemoglobin level (response rate of 20%). 13 out of 42 patients (30.9%) continued to receive Venofer® up to 8.
Thuốc truyền tĩnh mạch Venofer 20mg/ml ( lọ 5ml ) điều trị thiếu sắt
Venofer must only be administered intravenously either by slow injection or by infusion. The dosage of Venofer is expressed in terms of mg of elemental iron. Each mL contains 20 mg of elemental iron. The usual total treatment course of Venofer is 1000 mg. Venofer treatment may be repeated if iron deficiency reoccurs.

Venofer 20 mg Fe/ml 5X5 ml
Venofer Prices, Coupons and Patient Assistance Programs. Venofer (iron sucrose) is a member of the iron products drug class and is commonly used for Iron Deficiency Anemia.. The cost for Venofer intravenous solution (20 mg/mL) is around $332 for a supply of 25 milliliters, depending on the pharmacy you visit.

Venofer Injection 100 MG at best price in Chennai by Jain Pharma Surgicals ID 12932718088
Venofer (iron sucrose) +. betaxolol. 1 interaction. Monitor/Modify Tx. iron sucrose + betaxolol. monitor BP: combo may incr. risk of hypotension (additive effects) betaxolol ophthalmic. Find medical information for Venofer on epocrates online, including its dosing, contraindications, drug interactions, and pill pictures.
Venofer Inj 100mg 1Ampx5ml
For all patients, Venofer® doses should be prescribed on a high risk infusion chart. Venofer® should be given in 100ml of sodium chloride 0.9%. Only sodium chloride 0.9% should be used for flushing. No additional therapeutic agents should be added to the bag. Each Venofer® dose should be administered over 30 minutes.

VENOFER (IRON SUCROSE INJECTION,USP) 100mg ELEMENTAL IRON PER 5mL (20mg/mL)
Injectafer has an average rating of 7.4 out of 10 from a total of 8 ratings on Drugs.com. 71% of reviewers reported a positive effect, while 29% reported a negative effect. Venofer has an average rating of 5.1 out of 10 from a total of 39 ratings on Drugs.com. 44% of reviewers reported a positive effect, while 53% reported a negative effect.