
X.1.h. Elektron Valensi (Teori) YouTube
Introduction and objectives. A summary of this, and the other updates reported here, has been published earlier 1.This update begins with the consideration of classification of diabetic polyneuropathies (DPNs) and then provides definitions, minimal criteria for diagnoses, and estimation of severity of typical DPN, i.e. diabetic sensorimotor polyneuropathy (DSPN).

KI + KIO3 + H2SO4= K2SO4 + I2 + H2O как составить уравнение окислительновосстановительной
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. Since there is an equal number of each element in the reactants and products of KIO3 = K + IO3, the equation is balanced.

How to name KIO3 YouTube
Preceding the joint meeting of the 19th annual Diabetic Neuropathy Study Group of the European Association for the Study of Diabetes (NEURODIAB) and the 8th International Symposium on Diabetic Neuropathy in Toronto, Canada, 13-18 October 2009, expert panels were convened to provide updates on classification, definitions, diagnostic criteria, and treatments of diabetic peripheral neuropathies.

Samuel Valensi Forum • Culture et Économie
The atom I: went from (0) to ( + 5), it lost electrons, so it is oxidized. 2- Separate the redox reaction into two half-reactions: one for the oxidation, and one for the reduction. Reduction half: ( 0) IX2 + KOH K ( − 1) I Oxidation half: ( 0) IX2 + KOH K ( + 5) I O3 3- Balance all elements except oxygen and hydrogen in both half equations:

KIO3+NaHSO3 YouTube
Word Equation. Potassium Iodate = Potassium Iodide + Dioxygen. KIO3 = KI + O2 is a Decomposition reaction where two moles of aqueous Potassium Iodate [KIO 3] decomposes into two moles of aqueous Potassium Iodide [KI] and three moles of Dioxygen [O 2] gas. Show Chemical Structure Image.
/https://media.azurestandard.com/files/3a59edcf-0faf-44f9-9cdc-a0a6c08759fc)
Living Nutrition KIO3, Potassium Iodate Azure Standard
Potassium Iodate | KIO3 or IKO3 | CID 23665710 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological.

Standardization of Thiosulfate using KIO3 and Released Iodine YouTube
Cardiovascular Autonomic Neuropathy (CAN) Subcommittee of Toronto Consensus Panel on Diabetic Neuropathy worked to update CAN guidelines, with regard to epidemiology, clinical impact, diagnosis, usefulness of CAN testing, and management.
.jpg?mode=max)
Henry Valensi (French, 18831960)
Contoh: Unsur natrium dan kalium memiliki sifat yang sama karena masing-masing memiliki elektron valensi = 1. Suatu atom netral dapat melepaskan 1 atau lebih elektronnya dan membentuk ion yang bermuatan positif, atau menangkap elektron dan membentuk muatan negatif. Contoh: 2311 Na mempunyai 11 proton, 11 elektron, dan 12 netron.
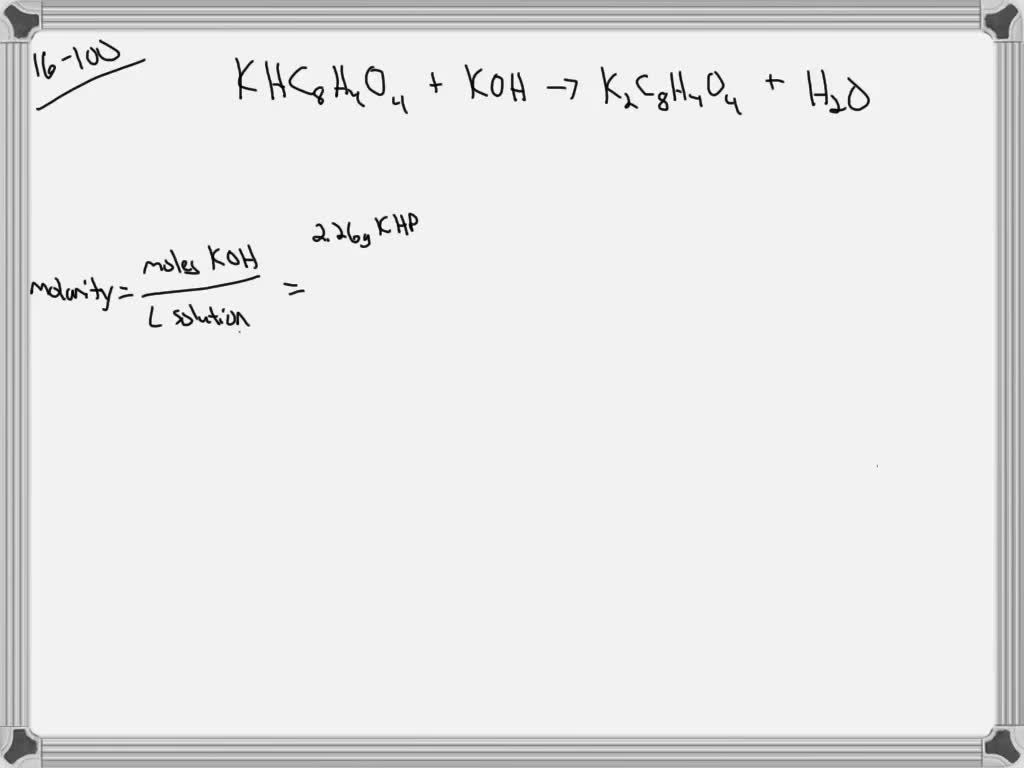
SOLVED An aqueous potassium iodate (KIO3) solution is made by dissolving 5.60×10^2 grams of
Potassium iodate is sometimes used for iodination of table salt to prevent iodine deficiency. In the US, iodized salt contains antioxidants, because atmospheric oxygen can oxidize wet iodide to iodine; other countries simply use potassium iodate instead. [5] Potassium iodate is also an ingredient in some baby formula. [6]

How to find the molecular mass of KIO3 (Potassium Iodate) YouTube
Molecular Formula:KIO3 Molecular Weight:214.00 Section 10 - Stability and Reactivity Chemical Stability: Stable under normal temperatures and pressures. Conditions to Avoid: High temperatures, dust generation. Incompatibilities with Other Materials: Reducing agents, combustible materials, flammable liquids..

Standardization of a solution of thiosulphate ions using KIO3 and KI No. 14 YouTube
Preceding the joint meeting of the 19th annual Diabetic Neuropathy Study Group of the European Association for the Study of Diabetes (NEURODIAB) and the 8th International Symposium on Diabetic Neuropathy in Toronto, Canada, 13-18 October 2009, expert panels were convened to provide updates on classification, definitions, diagnostic criteria, and treatments of diabetic peripheral neuropathies.

potassium iodate (KIO3)
STANDARISASI Natrium Tiosulfat ( Na2S2O3 ) Larutan Natrium tiosulfat dapat digunakan sebagai larutan baku sekunder untuk standarisasi iodium. Natrium tiosulfat dapat diperoleh dengan mudah dalam keadaan kemurnian yang tinggi, tetapi selalu ada ketidakpastian dari konsentrasi yang kita buat. Untuk itu sebelum digunakan larutan tiosulfat harus.

molecular mass of KIO3 Brainly.in
Cara lain untuk mencari elektron valensi suatu unsur adalah dengan sesuatu yang disebut konfigurasi elektron. Konfigurasi elektron mungkin terlihat rumit, tetapi ini hanyalah suatu cara untuk melambangkan orbital elektron dalam atom dengan huruf dan angka, dan cara ini mudah jika Anda mengetahui yang Anda kerjakan.
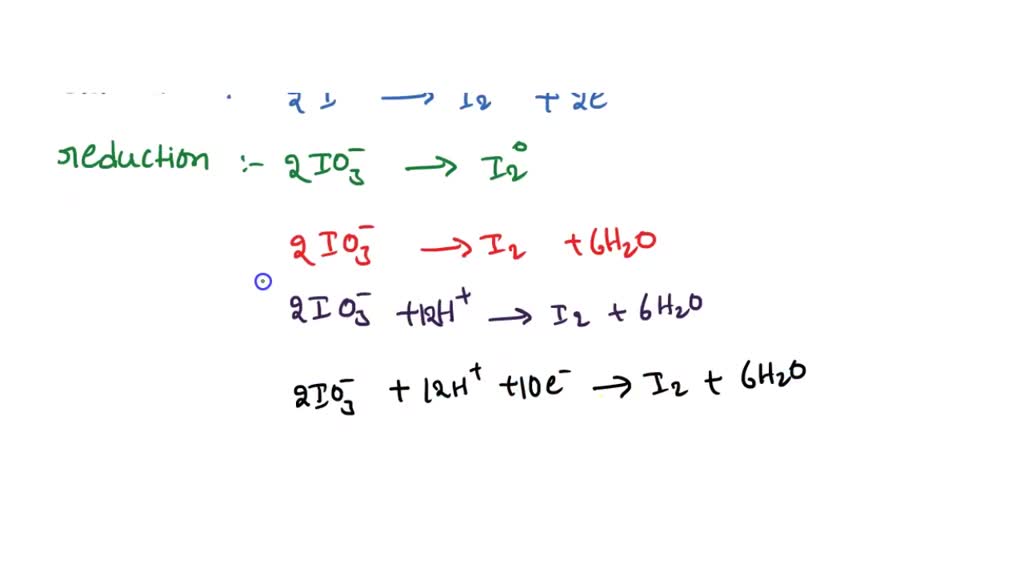
SOLVED Setarakan persamaan reaksi berikut menggunakan cara 1/2 reaksi ion elektron KIO3 + KI
Overall Chemical Equation Ensure that the elements on both sides of the molecular equation are balanced by using the online chemical equation balancer. KIO 3 = KI + O 3 How to Balance KIO3 = KI + O3 Solubility Equation Determine the state or phase of each substance (gas=g, liquid=l, solid/non-soluble=s, aqueous/soluble=aq) in its undissociated form by using the solubility rules or a solubility.

KIO3 + KI + H2SO4 = K2SO4 + I + H2O Método REDOX Respuesta YouTube
Potassium iodide (KI) and potassium iodate (KIO3) (from pills, liquids, or powders) can help protect the thyroid gland from the effects of radioactive iodine that can occur in the air, water, and foods after a nuclear event and accumulate in the thyroid and cause thyroid cancer. Be aware that these compounds do not protect other parts of the.

Epiphone Riviera Nick Valensi 08 Epiphone, Epiphone riviera, Riviera
KIO3 = K + IO3 is a redox reaction where O is oxidized and K, I are reduced. KIO 3 are reducing agents (i.e. they lost electrons) and KIO 3, KIO 3 are oxidizing agents (i.e. they gained electrons). Enter a redox reaction equation to balance it and calculate the reducing and oxidizing agents. Use uppercase for the first character in the element.