
Rx ItemTerconazole Vaginal 0.4 Cream 45Gm By Taro Pharma Gen Terazol
DESCRIPTION. Terconazole Vaginal Cream 0.4% is a white to off-white, water washable cream for intravaginal administration containing 0.4% of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl) -2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-isopropylpiperazine, compounded in a cream base consisting of.
Terazol 7 Cream Terazol 7 cream or generic terconazole is a prescription antifungal medicine
Terconazole is an anti-fungal cream and suppository used for treating vaginal yeast infections (Candida).It is related to several other anti-fungal drugs including fluconazole (), ketoconazole (), itraconazole (), miconazole (Micatin, Monistat), and clotrimazole (Lotrimin). It prevents growth of yeast by preventing production of the membranes that surround the yeast cells.

Terconazole Vaginal Cream 0.4, 45 g, Fougera (RX) Ingredients and Reviews
Terconazole suppositories should not be used together with vaginal contraceptive diaphragms that contain certain rubber or latex products. Side Effects. Along with its needed effects, a medicine may cause some unwanted effects. Although not all of these side effects may occur, if they do occur they may need medical attention.

Terconazole ClinicalInfo
Terconazole vaginal cream is an antifungal medication that treats yeast infections. A vaginal yeast infection causes burning, itching and changes to your vaginal discharge. To apply this medication, follow the instructions on the label. You can use the applicator to release the cream into your vagina as directed.

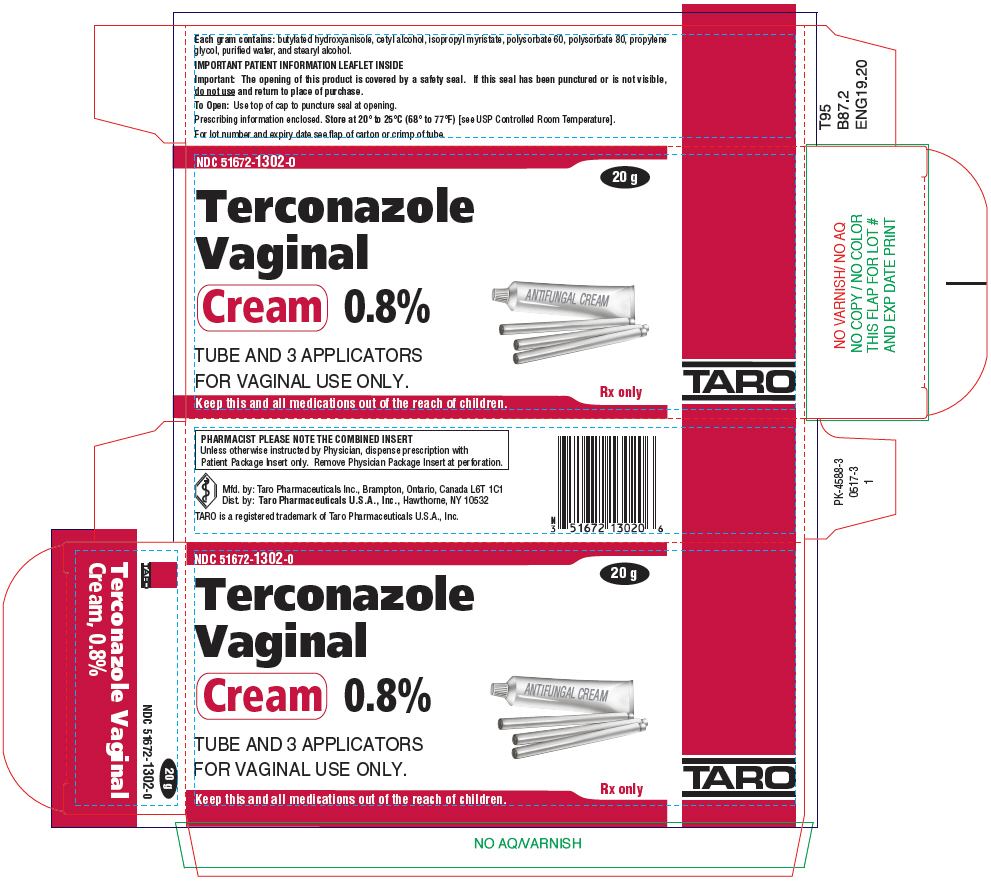
Terconazole Vaginal Cream 0.8, 20 g, Taro Pharmaceuticals (RX) Ingredients and Reviews
Mechanism of Action. Terconazole is a triazole ketal antifungal agent; involves inhibition of fungal cytochrome P450. Specifically, terconazole inhibits cytochrome P450-dependent 14-alpha-demethylase which results in accumulation of membrane disturbing 14-alpha-demethylsterols and ergosterol depletion.

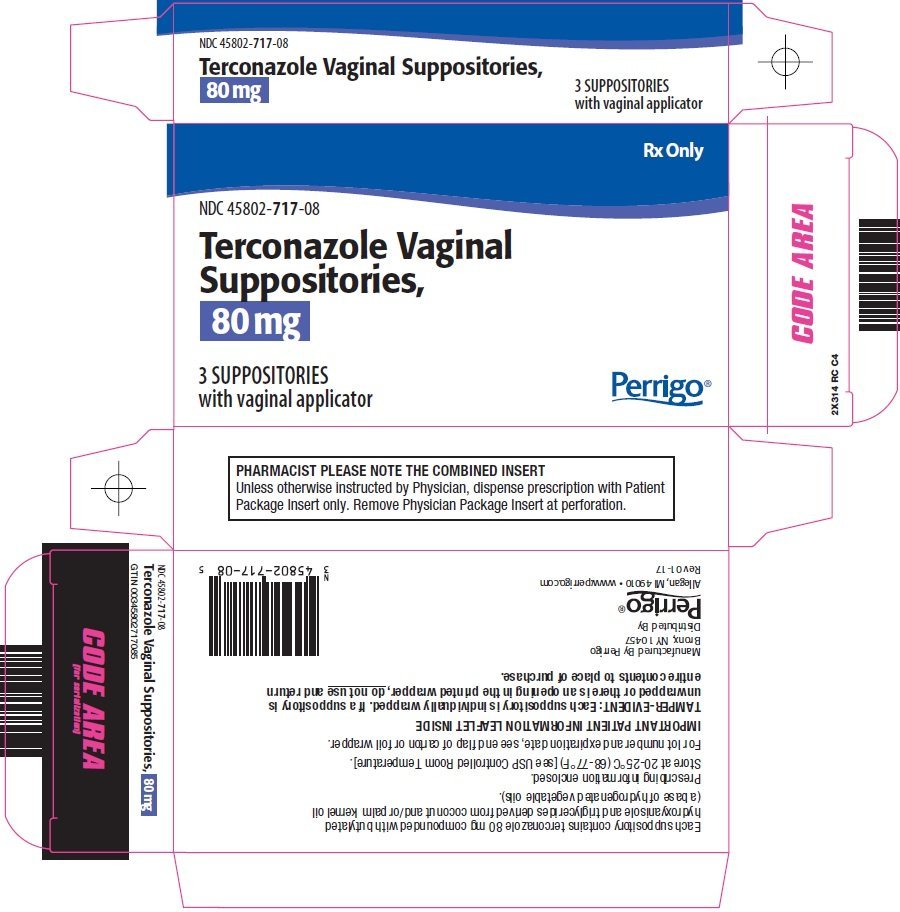
Terconazole Vaginal Suppositories 80 mg
Terconazole is the generic name for Terazol. It is a triazole antifungal drug that was approved by the FDA in 1987. Unlike miconazole, terconazole can only be obtained with a prescription from a doctor. It is available in a 0.4% and 0.8% vaginal cream as well as an 80 mg vaginal suppository. Miconazole —also known by its brand name, Monistat.

Terconazole 80 mg Suppository Blister Pack 3 Suppositories
Terconazole is an antifungal drug used in the treatment of vulvovaginal candidiasis. Terconazole is an anti-fungal drug that is mainly used to treat vaginal yeast infections (or vaginal candidiasis). It is classified as a triazole ketal derivative. 2 Terconazole was initially approved by the FDA in 1987. Label This drug is available in cream.
Terconazole FDA prescribing information, side effects and uses
Terazol 7 Description. TERAZOL ® 7 (terconazole) Vaginal Cream 0.4% is a white to off-white, water washable cream for intravaginal administration containing 0.4% of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2-(1 H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-isopropylpiperazine, compounded in a cream base consisting of butylated hydroxyanisole, cetyl.

GYNOCONAZOL 80 MG ( TERCONAZOLE ) 3 VAGINAL SUPPOSITORIES
Description for Terazol 3, Terazol 7. TERAZOL® 7 (terconazole) Vaginal Cream 0.4% is a white to off-white, water washable cream for intravaginal administration containing 0.4% of the antifungal agent terconazole, cis-1-[p-[[2(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4isopropylpiperazine, compounded in a cream base consisting of butylated.

GYNOCONAZOL 0.4 ( TERCONAZOLE ) VAGINAL CREAM 30 GM
Terconazole vaginal cream 0.4% is a white to off-white, water washable cream for intravaginal administration containing 0.4% of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2-(1 H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-isopropylpiperazine, compounded in a cream base consisting of butylated hydroxyanisole, cetyl alcohol, isopropyl myristate.

TERCONAZOLE VAGINAL CREAM 0.4
Terconazole is an antifungal prescription medicine approved by the U.S. Food and Drug Administration (FDA) for the treatment of vulvovaginal candidiasis. Vulvovaginal candidiasis can be an opportunistic infection (OI) of HIV. An OI is an infection that occurs more frequently or is more severe in people with weakened immune systems —such as.

Terconazole cream/ suppository Drug Details
Wash your hands before and after using this medicine. Remove the cap from the end of the tube. Screw the open end of the applicator onto the tube of cream. Squeeze the tube and fill the applicator until it is full or the plunger stops. Unscrew the applicator from the tube and replace the cap on the tube.

Rx ItemTerconazole 80Mg Suppository 3 By Perrigo Pharma gen Terazol
Terconazole. Terconazole is an antifungal drug used to treat vaginal yeast infection. It comes as a lotion or a suppository and disrupts the biosynthesis of fats in a yeast cell. It has a relatively broad spectrum compared to azole compounds but not triazole compounds. Testing shows that it is a suitable compound for prophylaxis for those that.

Terconazole ClinicalInfo
Terconazole vaginal may cause serious side effects. Call your doctor at once if you have: new or worsening symptoms; fever, chills, flu symptoms; severe vaginal irritation; or. severe skin reaction--fever, sore throat, swelling in your face or tongue, burning in your eyes, skin pain, followed by a red or purple skin rash that spreads.

Terconazole Vaginal Cream _ 0.8 Tube 20gm/tb Medex Supply
Terconazole - Clinical Pharmacology. Absorption - Following a single intravaginal application of a suppository containing 240 mg 14 C-terconazole to healthy women, approximately 70% (range: 64-76%) of terconazole remains in the vaginal area during the suppository retention period (16 hours); approximately 10% (range: 5-16%) of the administered radioactivity was absorbed systemically over 7 days.

Terconazole Vaginal Cream FDA prescribing information, side effects and uses
Terconazole - Last updated on December 12, 2022 All rights owned and reserved by Memorial Sloan Kettering Cancer Center. Last Updated. Monday, December 12, 2022. Add Resources to Your List. Terconazole. Educational Resources. Log in to print or send this list to your patient and save lists of resources you use frequently.