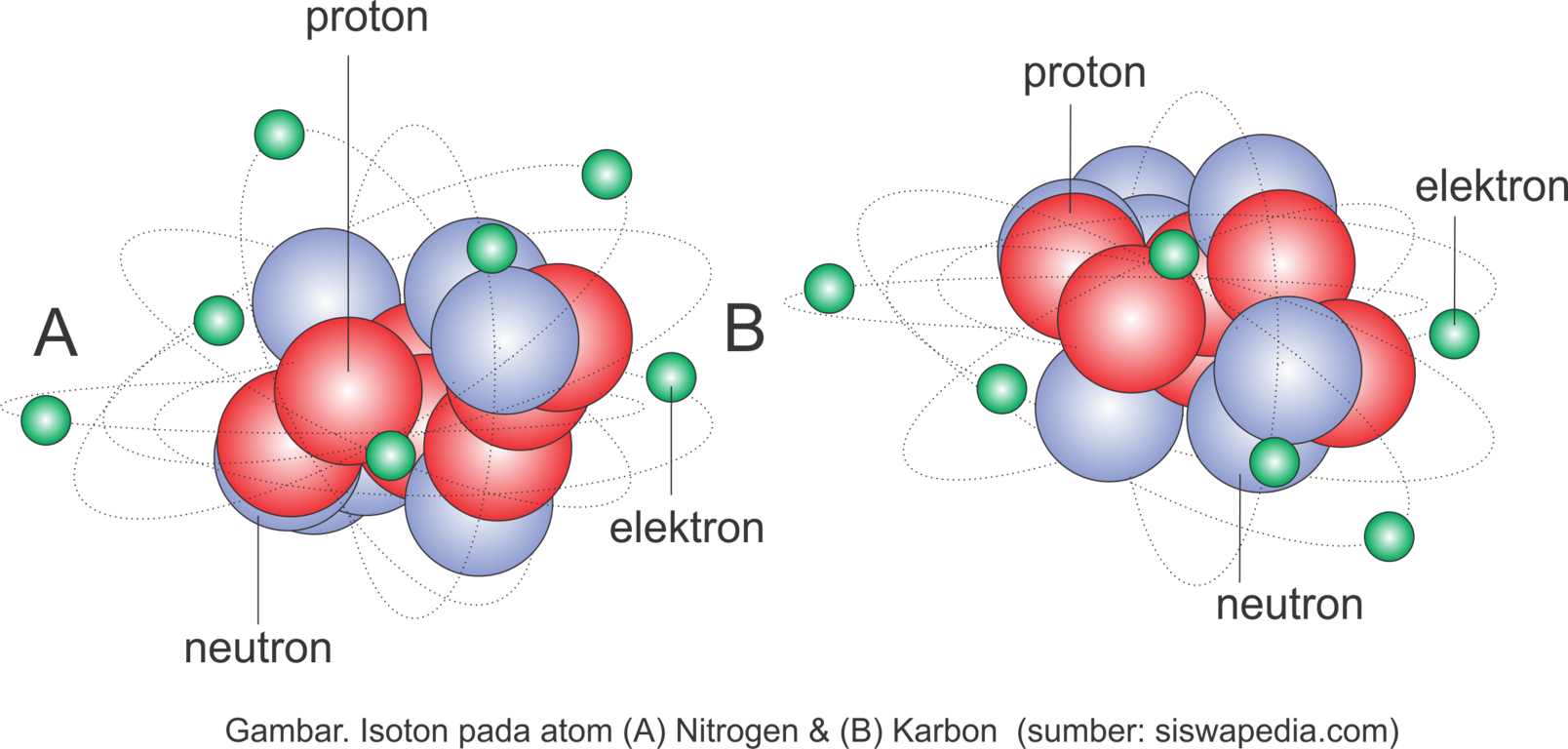
Pengertian Serta Contoh Isotop, Isobar dan Isoton Siswapedia
Model/Teori Atom Niels Bohr: Pengertian, Gambar, Kekurangan dan Kelebihannya. Susunan Atom dan Cara Menentukan Jumlah Proton, Elektron & Neutron. Isobar adalah unsur yang memiliki nomor massa sama tetapi nomor atomnya berbeda dengan kata lain unsur yang berbeda jenis tetapi nomor massanya sama.

10 Contoh Soal Kimia Isotop Isobar dan Isoton beserta Jawabannya Materi Kimia
Isotopes and Isobars. Isobar are elements that differ in chemical properties but have the same physical property. So, we can say that isobars are those elements that have a different atomic number but the same mass number. In contrast, Isotopes are those elements having the same atomic number and different mass numbers.

Cara Menentukan Isotop Isobar Dan Isoton Studyhelp
AMS Weather Studies CWS 6 - 2 - FL22 rigure 1. Analyzed weather map with isobars, radar, and data for 12∠25 SEP 2022. [NOAA Weather Prediction Center]. In this study, we focus mostly on the Great Lakes, Middle Atlantic, and Northeast states. Known for cold weather in winter with bouts of snow during the early autumn season, Michigan was the.
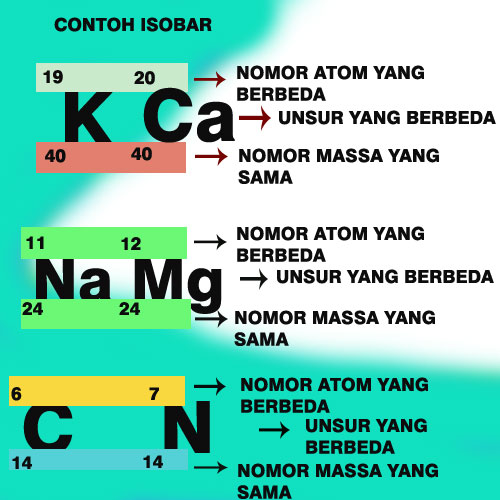
Panduan Pengertian Dan Cara Memilih Isotop,Isobar,Isoton Dan Isoelektron Atap Ilmu
Isobars are elements that have the same number of nucleons (sum of protons and neutrons). The series of elements with 40 Mass numbers serve as a good example; 40 16 S, 40 17 Cl, 40 18 Ar, 40 19 K, and 40 20 Ca. The nucleus of all the above-mentioned elements contain the same number of particles in the nucleus but contain varying numbers of.
Pengertian Nomor Atom, Nomor Massa, Isotop, Isobar, Isoton, Waktu Paruh dan Sistem Periodik
Service was great and so was the ambiance!" Top 10 Best Farm to Table Restaurants in Buffalo, NY - November 2023 - Yelp - The Dapper Goose, SZND, Prescott's Provisions, The Little Club, Share Kitchen & Bar Room, CRāVing Restaurant, Iron Tail Tavern, The Grange Community Kitchen, Marble + Rye, Rick's On Main.

Kumpulan Contoh Soal Isotop, Isobar, dan Isoton Kimia Kelas 10 CoLearn halaman 5
Iodine isobars are used to treat goiter. Cobalt isobars can be used to treat cancer. Frequently Asked Questions. Q1. What are isotopes and isobars? Answer. Isotopes are atoms of the same element with the same atomic number but a different mass number. Isobars are atoms of various elements that have the same mass number but a different atomic.
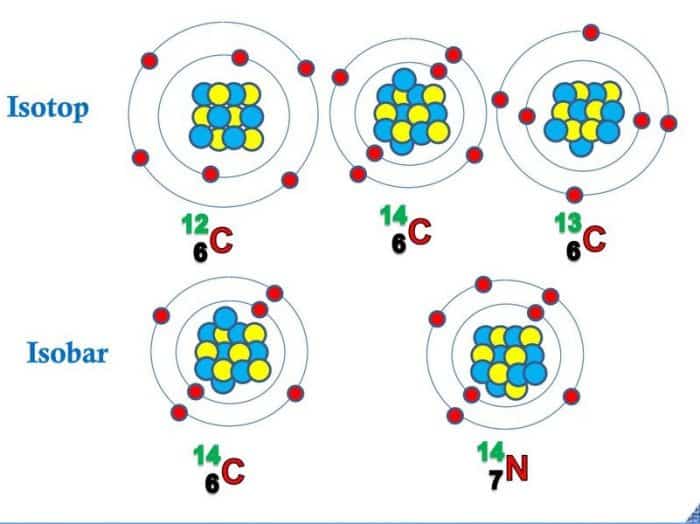
Arti Isotop Isobar Dan Isoton
Isobars, Isotopes and Isotones. Isotopes are atoms having same atomic number (Protons) but different mass number. i.e., the number of neutrons are different. Hence the atomic weights of the isotopes of an element are different. Isotopes of the same element have the same chemical properties because they have the same number and arrangement of.

Cara Menentukan Isotop Isobar Dan Isoton Studyhelp
The terms isotopes, isobars, and isotones are used to describe the interactions between the atoms of various chemical elements. The concept of the nucleus was discovered by Rutheford in his atomic model popularly known as Rutherford's atomic model, which states that ' Protons and neutrons, which constitute almost all of the mass of the.
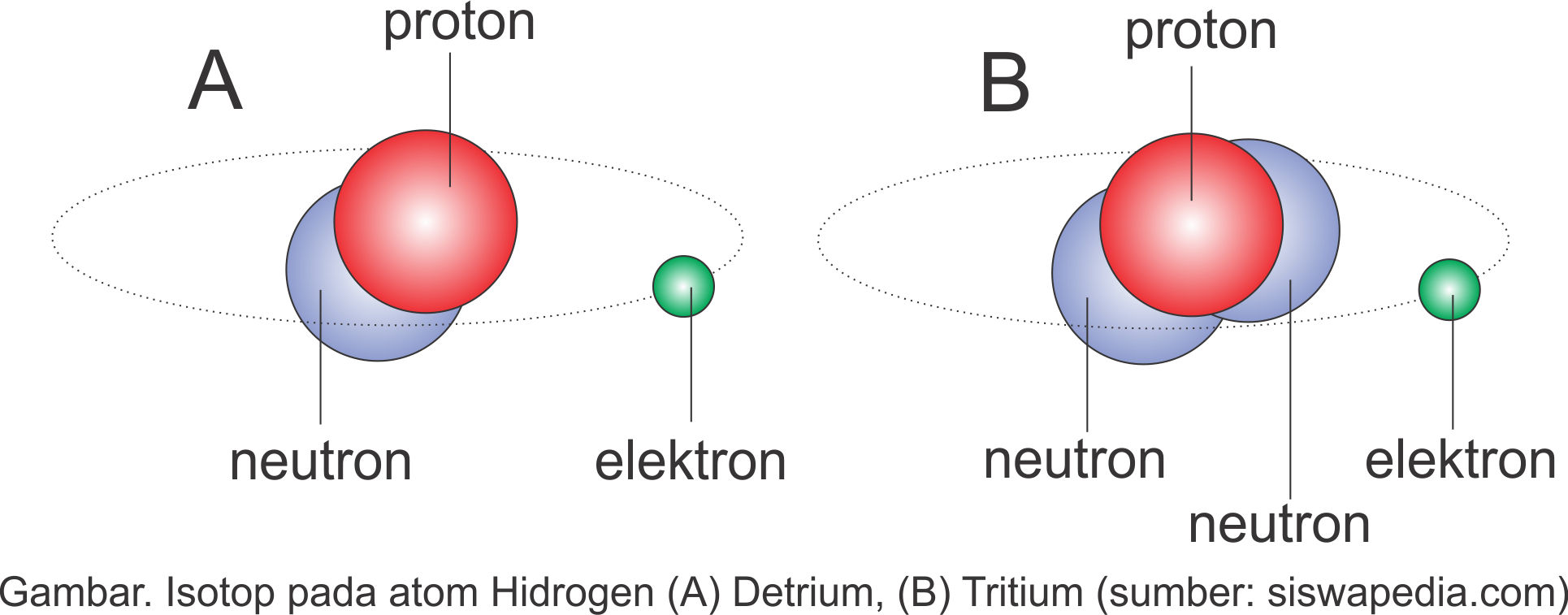
Pengertian Serta Contoh Isotop, Isobar dan Isoton Siswapedia
- Isobars are atoms that have the same mass number but different atomic numbers are called isobars. - The word isobar meaning 'equally heavy' is taken from the Greek isos = equal, and barys = heavy. Examples of isobars - For example, 40 Ar 18, 40 K 19, and 40 Ca 20 are isobaric atoms. - Similarly, 235 U 92, 235 Np 93, 235 Pu 94 are.

Perbedaan Isotop, Isobar, Isoton, dan Isomer Muhyidin, SKM
Isobars Elements having the same mass number (A) but different number of protons (Z) are isobars. Example: 40 16 S, 40 17 Cl, 40 18 Ar, 40 19 K, and 40 20 Ca. Isotones Elements having the same number of neutrons (N) but a different number of protons (Z) or mass number (A) are isotones.
Isotop, Isobar and Isoton (Kimia SBMPTN, UN, SMA) YouTube
Tabel isotop di bawah menunjukkan isotop unsur kimia, termasuk seluruh isotop dengan waktu-paruh sekurang-kurangnya satu hari. Tabel disusun berdasarkan kenaikan nomor atom dari kiri ke kanan dan kenaikan jumlah neutron dari atas ke bawah.. Warna sel menunjukkan waktu-paruh masing-masing isotop; jika bergaris-tepi, warnanya menunjukkan waktu-paruh dari isomer inti atom yang paling stabil.

Hubungan yang benar mengenai isotop, isoton dan isobar de...
Memahami Definisi Isotop, Isobar dan Isoton dalam Ilmu Kimia Beserta Contoh. 27 Oktober 2023. Yusuf Abdhul Azis. Pada saat pertama kali mengenal istilah isotop, isobar, dan juga isoton ini mungkin kamu akan sedikit bingung bahkan pusing. Apalagi ada sedikit perbedaan antara isotop, isobar dan juga isoton. Meskipun begitu, dewasa ini proses.
Kelompokkan Atom Atom Berikut Ke Dalam Isotop Isobar Dan Isoton Image Sites
Penjelasan Isotop Isobar Isoton Isotop Isobar Isoton merupakan bahasan yang mengulas atom yang memiliki kriteria tertentu, seperti nomor atom sama, nomor massa sama, atau atom dengan jumlah neutron sama. Nomor atom menunjukkan jumlah proton yang terdapat pada suatu unsur. Sedangkan nomor massa ditentukan oleh jumlah proton dan neutron. Umumnya, jumlah neutron dalam inti suatu atom […]

Isotop Isobar Isoton Pengertian, Penjelasan, Contoh, Soal dan Jawaban
Untuk lebih lanjutnya akan dipelajari materi pokok terkhusus pada pengelompokan unsur-unsur radioaktif yaitu isotop isobar dan isoton. Perhatikanlah data pada tabel berikut! No Isotop Isobar Isoton . 1 2 C. Pertanyaan 1. Berdasarkan peneglompokan unsur-unsur pada tabel di atas simpulkan apa yang dimaksud dengan isotop, isobar dan isoton?

Struktur Atom Kimia Kelas 10 • Part 3 Isotop Isobar Isoton, Massa Atom & Molekul Relatif YouTube
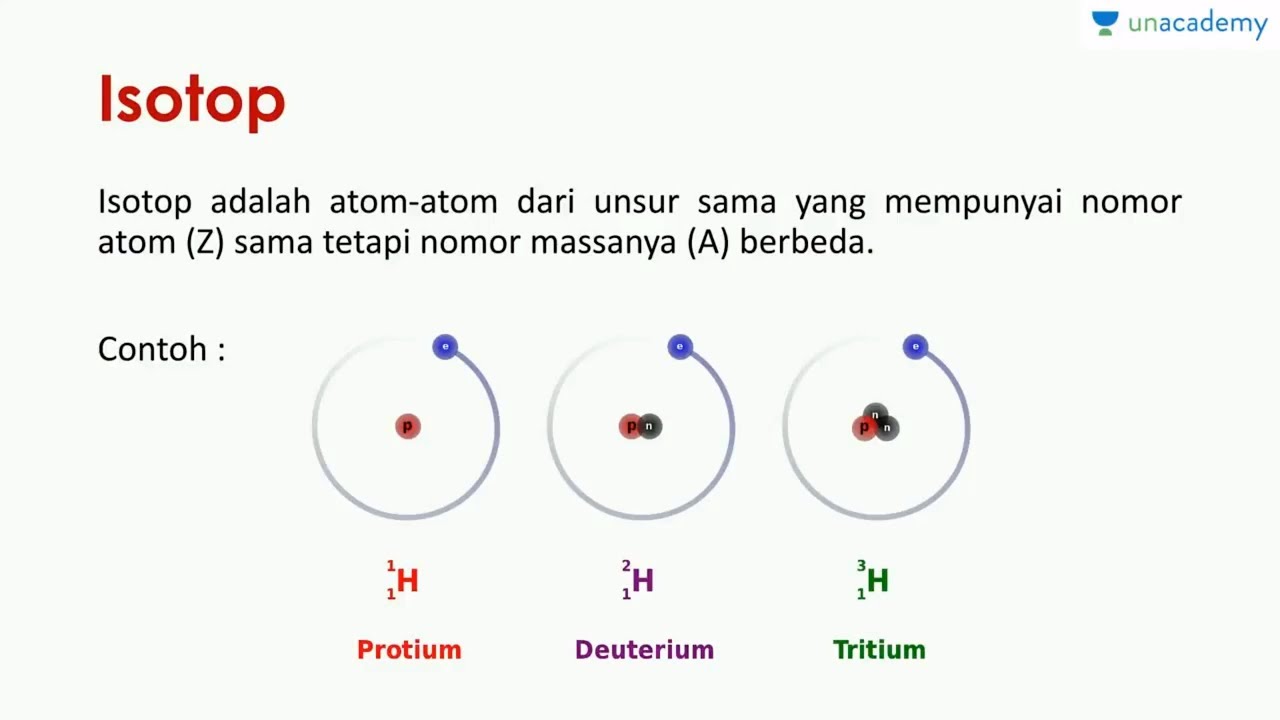
Isotopes. Isotopes are atoms of an element which have the same proton number but different nucleon numbers. Example: Hydrogen is the common example which has three isotopes. These have the same atomic number, one, but different mass numbers 1, 2, and 3. These three isotopes are commonly known as hydrogen or protium, deuterium (D) and tritium (T.

Pembahasan Soal Isotop, Isobar, dan Isoton Struktur Atom YouTube
Jakarta -. Berdasarkan nomor atom dan nomor massanya, atom-atom dari unsur yang sama ataupun berbeda dapat dibedakan menjadi isotop, isobar, dan isoton. Seperti diketahui, dalam ilmu kimia, kita diperkenalkan dengan konsep atom yaitu bagian terkecil dari suatu materi. Partikel dasar penyusun atom adalah proton, elektron, dan neutron.