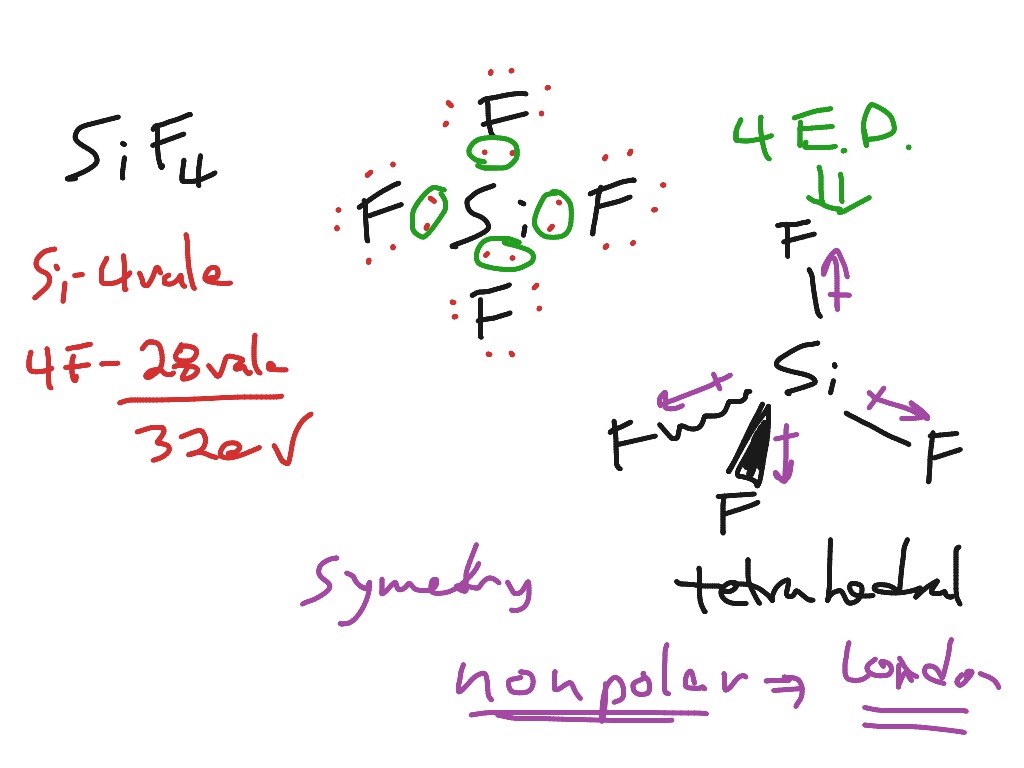
Lewis Structure For Sif4
Electronegative difference calculation SiF4 molecule: To sketch the SiF4 Lewis structure by following these instructions: Step-1: Adding valence electron on the silicon atom. Step-2: Adding valence electron on fluorine atom in the SiF4 molecule. Step-3: Combining step1 and step2 to get step3 for SiF4 dot structure.
Lewis Structure Of Sif4
Chemistry questions and answers. Draw the Lewis structure for SiF4; arrangement of atoms shown below, dashed lines show connections between atoms. F.SI-F **SHOW WORK** This question is worth a total of 6 points; 1 point for the correct selections (assessed when you answer) and 5 points for the Lewis structure on your work (assessed when I.

Sif4 Lewis Structure
SIF4 is a covalent compound, which consists of silicon and fluorine atoms. It is named tetrafluorosilane or silicon tetrafluoride. The melting and boiling point of silicon tetrafluoride is -95.0 °C and -90.3 °C and hence, it exists as a gas at room temperature. Silicon tetrafluoride is a colorless, toxic, corrosive, and non-flammable gas with.

Lewis Structure Of Sif4
SiF4 is non-polar. The compound occurs as great example of non-polar compound, which is quite attainable for showing the exceptional compound in the series of the polar compounds. Silicon tetrafuloride has polar bonds between Silicon and fluorine but the tetrahedral shape eliminates the dipole movement of four Si-F sigma bonds.

Sif4 Lewis Structure
Steps. To properly draw the SiF 4 Lewis structure, follow these steps: #1 Draw a rough sketch of the structure. #2 Next, indicate lone pairs on the atoms. #3 Indicate formal charges on the atoms, if necessary. Let's break down each step in more detail.

Sif4 Lewis Structure
Steps of drawing SiF4 lewis structure Step 1: Find the total valence electrons in SiF4 molecule. In order to find the total valence electrons in a SiF4 molecule, first of all you should know the valence electrons present in silicon atom as well as fluorine atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.). Here, I'll tell you how you can easily.

Estructura Lewis Sif4 2020 idea e inspiración
Key Points To Consider When drawing The SiF4 Molecular Geometry. A three-step approach for drawing the SiF4 molecular can be used. The first step is to sketch the molecular geometry of the SiF4 molecule, to calculate the lone pairs of the electron in the central silicon atom; the second step is to calculate the SiF4 hybridization, and the third step is to give perfect notation for the SiF4.
Sif4 Lewis Structure
SIF4 lewis structure octet rule. In the above Lewis structure, silicon and fluorine do not have any charges and the central silicon atom completes its octet therefore this structure is a stable Lewis structure.For a stable Lewis structure, all the atoms in the molecules present must satisfy the octet rule, octet rule states that to attain a stable configuration valence shell of an atom.

SiF4 Lewis Structure, Molecular Geometry, Hybridization, and Polarity Techiescientist
2024-02-17. Description. Silicon tetrafluoride appears as a colorless, nonflammable, corrosive and toxic gas with a pungent odor similar to that of hydrochloric acid. Very toxic by inhalation. Vapor is heavier than air.

SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Techiescientist
The Lewis electron structure for the NH 4+ ion is as follows: The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Using Equation 8.5.1, the formal charge on the nitrogen atom is therefore. formal charge(N) = 5 − (0 + 8 2) = 0.
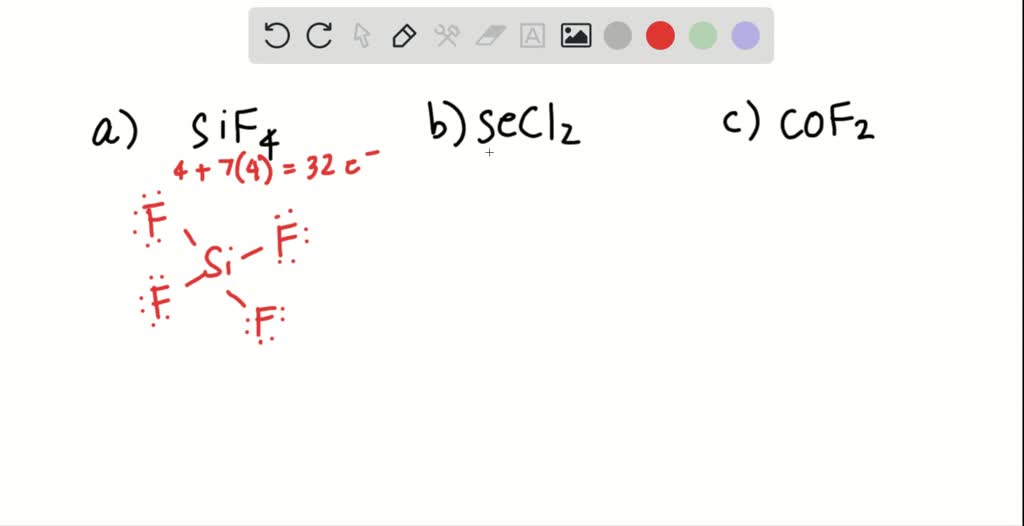
SOLVED Draw a Lewis structure for (a) SiF4; (b) SeCl2; (c) COF2 (C is central).
Lewis Structure Finder. This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
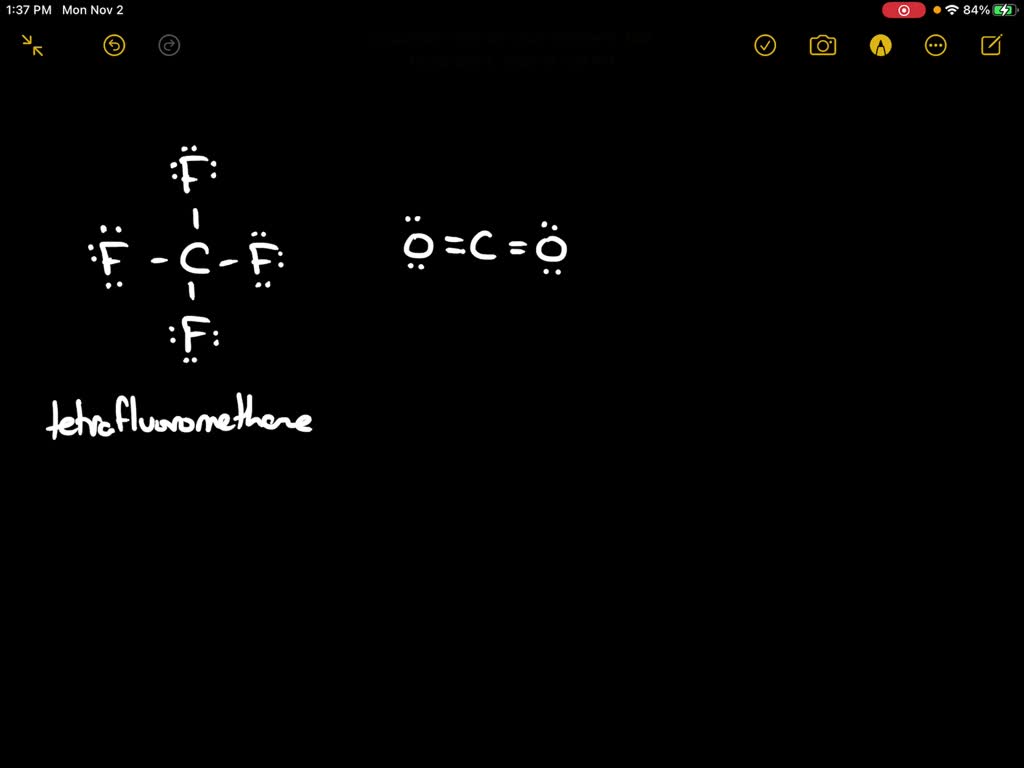
Draw the Lewis structure for SiF4; arrangement of ato… SolvedLib
This video shows you how to draw the lewis dot structure for SiF4 (silicon tetrafluoride). The video also discusses the molecular geometry, bond angle, and if SiF4 is polar or nonpolar.

Tetrahedral Angle 3d Balls Sif4 Lewis Structure Molecular Geometry, HD Png Download vhv
A step-by-step explanation of how to draw the SiH4 Lewis Dot Structure (Silicon Tetrafluoride).For the SiH4 structure use the periodic table to find the tota.

SiF4 Molecular Geometry Science Education and Tutorials
Transcript: All right: this is Dr. B. We're going to do the Lewis structure for SiF4. So let's start out. On the periodic table, Silicon is in group 4, sometimes called 14, so it's got 4 valence electrons. Fluorine, group 7--7 valence electrons, but we have 4 of those so we're going to multiply that by 4; and that is 4 plus 28, is 32. So we.

How to draw SiF4 Lewis Structure? Science Education and Tutorials
Step #1: Calculate the total number of valence electrons. Here, the given molecule is SiF4 (silicon tetrafluoride). In order to draw the lewis structure of SiF4, first of all you have to find the total number of valence electrons present in the SiF4 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).
Lewis Structure For Sif4
October 15, 2023 by Deep. The information on this page is fact-checked. Lewis structure of SiF 4. The Lewis structure of SiF4 contains four single bonds, with silicon in the center, and four fluorines on either side. There are three lone pairs on each fluorine atom, and the silicon atom does not have any lone pair.