NH3 BF3 Lewis Structure
Dalam molekul NH 3 terdapat sepasang elektron yang tidak digunakan (elektron bebas) sehingga disebut Pasangan Elektron Bebas (PEB). Tiga pasang elektron yang digunakan bersama oleh atom N dan atom H disebut Pasangan Elektron Ikatan (PEI). 2. Struktur Lewis Molekul H 2 O. Atom 8 O memiliki konfigurasi elektron 8 O:2, 6.

Estructura de Lewis NH3, Amoniaco » Quimica Online
Steps of drawing lewis structure of BF 3. There are general guidelines to draw a lewis structure step by step and they are mentioned below. In this lesson, we use those rules to draw the BF 3 lewis structure and they are explained in detail in next sections of this tutorial. If you are are beginner to lewis structure drawing, follow these sections slowly and properly to understand.

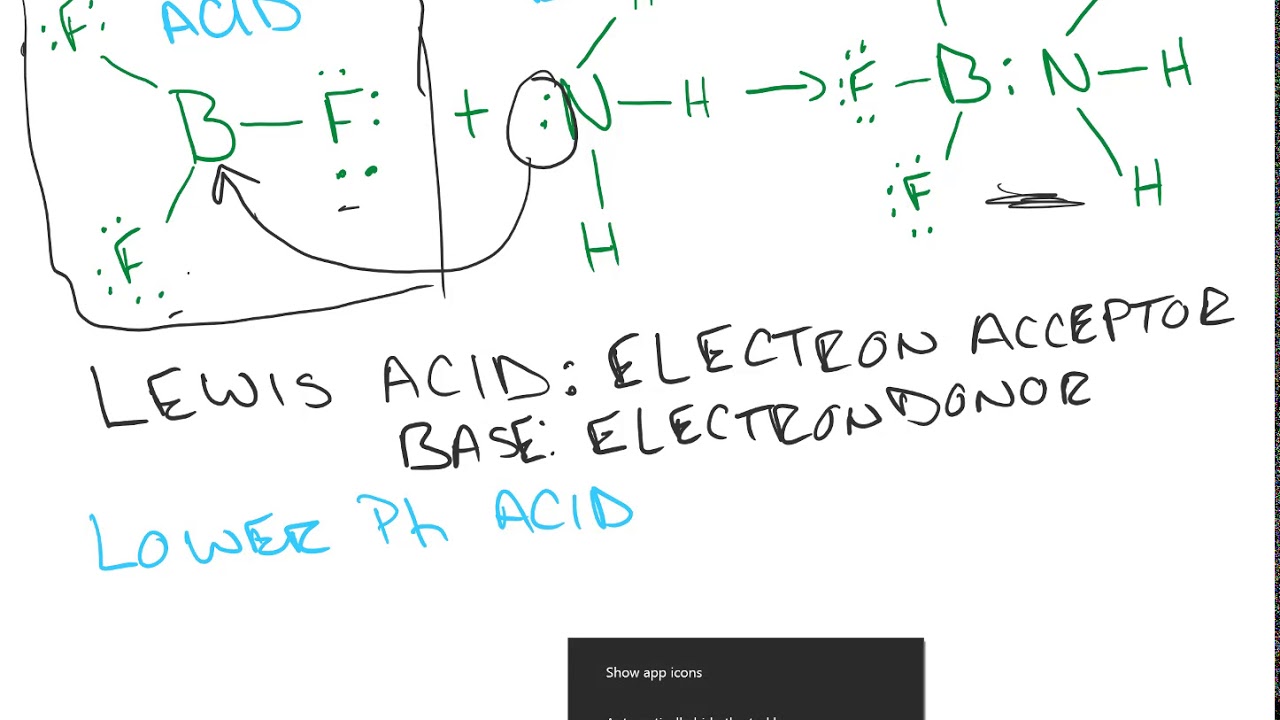
NH3 And BF3 form an adduct readily because they form
22. Organic Chemistry ( 0) 23. Chemistry of the Nonmetals ( 0) 24. Transition Metals and Coordination Compounds ( 0) Ammonia reacts with BF3 to form the adduct BF3-NH3. Identify the Lewis acid in this reaction.

Buatlah Struktur Lewis Nh3
Explanation: The structure of H3N-BF3 is. The B and N atoms each have four single bonds, so their hybridizations are sp3 with bond angles of 109.5°. Answer link. Every bond angle is approximately 109.5°. > The structure of "H"_3"N-BF"_3 is The "B" and "N" atoms each have four single bonds, so their hybridizations are "sp"^3 with bond angles.

Struktur Lewis senyawa NH3BF3 adalah sebagai berikut H F...
Beranda. Perhatikan rumus struktur Lewis dari NH 3 BF 3 ber. Iklan. Pertanyaan. Perhatikan rumus struktur Lewis dari NH 3 BF 3 berikut! Pasangan elektron yang membentuk ikatan kovalen koordinasi ditunjukkan oleh nomor. (Nomor atom H = 1; N = 7; B = 5; F = 9) 1. 2.

Gambarkan Struktur Lewis Senyawa Nh3 Ilmusosial & Pendidikan
Consider the following acid-base reaction:NH3 + BF3 ⇌ H3N+—BF3−1. For all the reactants and products, draw Lewis structures.2. Identify the nucleophile (base) and electrophile (acid) in the reaction.3. Draw curved arrows to show the flow of electrons.4. Determine if the reaction can be termed a Brønsted-Lowry acid-base reaction.

Ácidos y bases. Teoría de Lewis Física Química
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

Gambarkan Struktur Lewis Nh3
Ammonia Trifluoroborane or NH 3 BF 3 are a chemical compound. Let us study the fact about NH 3 BF 3 in more detail. Ammonia Trifluoroborane (NH3BF3) belongs to the borane-nitrogen-hydride family. The molecular weight of NH3BF3 is 84.84g/mol. Azaniumyl (trifluoro)boranuide is the IUPAC name for NH3BF3. ad.

Buatlah Struktur Lewis Nh3
The Lewis electron structure for the NH 4+ ion is as follows: The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Using Equation 4.4.1, the formal charge on the nitrogen atom is therefore. formalcharge(N) = 5 −(0 + 8 2) = 0.

NH3 And BF3 form an adduct readily because they form
Soal No. 1 Apa yang dimaksud dengan struktur Lewis? Pembahasan: Struktur lewis adalah penggambaran elektron valensi suatu atom dengan notasi (penulisan lambang atom dikelilingi oleh titik di sekitarnya). Soal No. 2 Gambarkan konfigurasi elektron dan struktur Lewis unsur-unsur di bawah ini! 11Na 6C 8O 17Cl 12Mg Pembahasan: 1. Konfigurasi elektron 11Na = 2, 8, 1
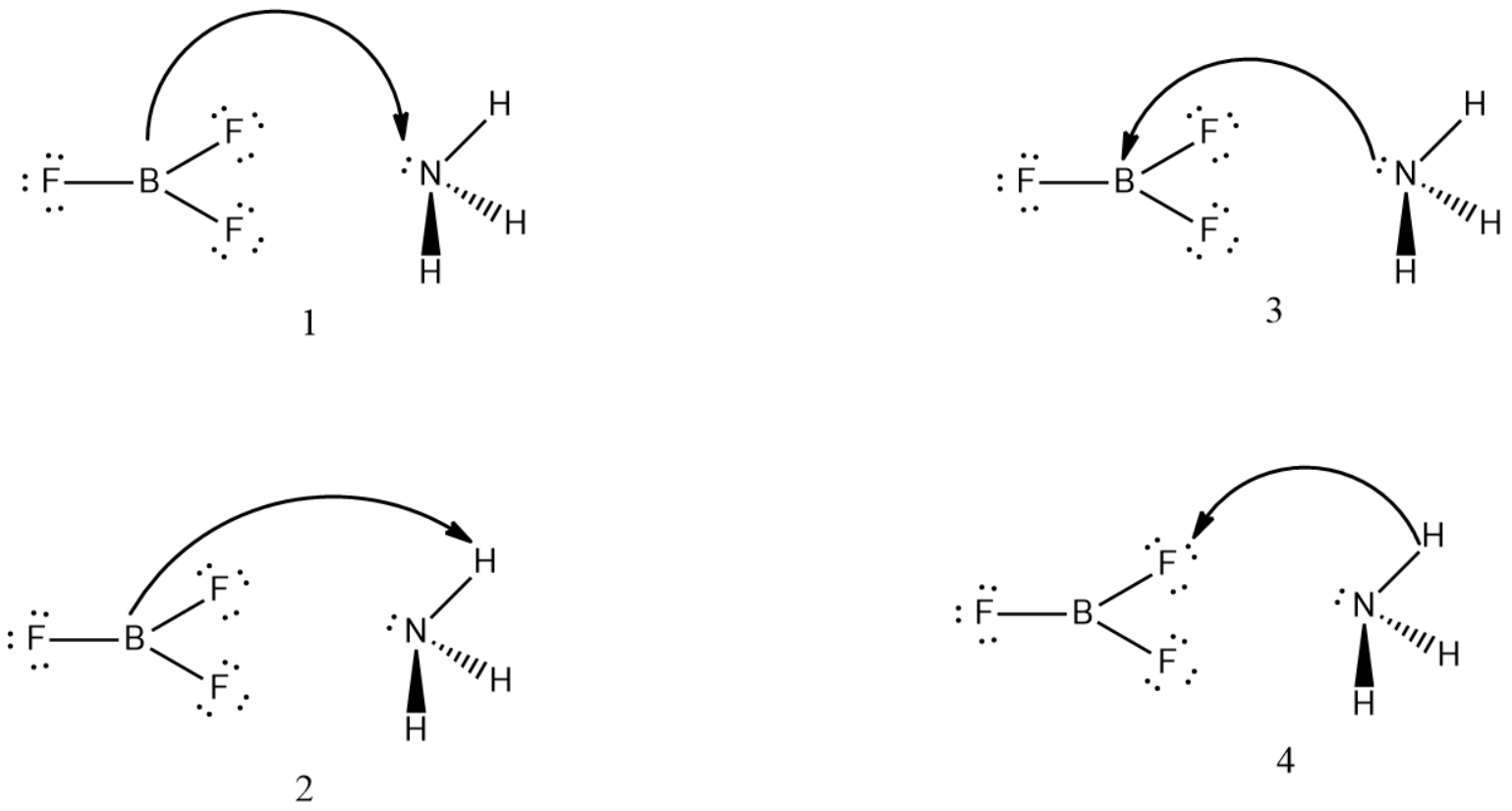
{ BF }_{ 3 } and { NH }_{ 3 } undergo Lewis acidbase reaction forming an adduct. Which
Section 7: Extensions of the Lewis Structure Model. Page ID. With these thoughts in mind, we turn to a set of molecules which challenge the limits of the Lewis model in describing molecular structures. First, we note that there are a variety of molecules for which atoms clearly must bond in such a way as to have more than eight valence electrons.

Estrutura De Lewis Nh3
Pertanyaan. Perhatikan rumus struktur Lewis senyawa NH 4 Cl berikut! Ikatan kovalen koordinasi pada gambar tersebut ditunjukkan nomor. (Nomor atom N = 7; H = 1; Cl = 17) 1. 2. 3.

Struktur Lewis Senyawa Nh3Bf3 Sebagai Berikut Extra
A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of vale.
Molekul NH3BF3 terbentuk ketika amonia direaksikan...
Boron Trifluoride (BF3) is an inorganic compound as it lacks a carbon atom or C-H bond in the molecule. Manufactured from the reaction of boron oxides and hydrogen fluoride, the chemical compound BF3 has a pungent smell and is colorless in nature. The compound behaves differently in different states of matter.

Lewis Dot Structure For Bf3
NH3 Lewis Structure, Geometry, and Hybridization. Ammonia is the simplest binary hydride made up of nitrogen and hydrogen denoted by its chemical formulae as NH3. It is a stable pnictogen hydride where all the atoms are covalently bonded to achieve a reactive state. Ammonia is lighter than the air, colorless, and pungent in smell.

BF3 Lewis Structure (Boron Trifluoride) YouTube
BF3 Hybridization. Hybridization stands for mixing atomic orbitals into new hybrid orbitals. They are accommodating to explain molecular geometry and nuclear bonding properties. There are several types of hybridization like SP3, SP2, SP. BF3 is SP2 hybridization. For this molecule, It is SP2 because one π (pi) bond is required for the double.