
Nh3 Estrutura De Lewis AskSchool
To sketch the HBr Lewis structure by following these instructions: Step-1: HBr Lewis dot Structure by counting valence electrons on the bromine atom. Step-2: Lewis Structure of HBr for counting valence electrons around the terminal hydrogen atoms. Step-3: Lewis dot Structure for HBr generated from step-1 and step-2.
[Solved] draw a lewis structure for the followings HBr C2HCI Course Hero
Struktur Lewis HBr (hidrogen bromida) memiliki atom hidrogen (H) dan atom brom (Br) yang mengandung ikatan tunggal di antara keduanya. Terdapat 3 pasangan elektron bebas pada atom brom (Br). Jika Anda tidak memahami apa pun dari gambar struktur Lewis HBr (Hidrogen bromida) di atas, ikuti terus saya dan Anda akan mendapatkan penjelasan detail.
PPT Covalent Compounds PowerPoint Presentation, free download ID2170124
Lewis Structure Finder. This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

How to draw HBr Lewis Structure? Science Education and Tutorials
Here's how you can easily draw the HBr Lewis structure step by step: #1 Draw a rough skeleton structure. #2 Mention lone pairs on the atoms. #3 If needed, mention formal charges on the atoms. Now, let's take a closer look at each step mentioned above.

Hydrogen bromide (HBr) molecule. Skeletal formula Stock Vector Image & Art Alamy
Step #1: Calculate the total number of valence electrons. Here, the given molecule is HBr (hydrogen bromide). In order to draw the lewis structure of HBr, first of all you have to find the total number of valence electrons present in the HBr molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).

Soal Gambarkan struktur lewis dari senyawa(!) a. HBr(ArH=1;Br=35)
A step-by-step explanation of how to draw the HBr Lewis Dot Structure (Hydrogen bromide).For the HBr structure use the periodic table to find the total numbe.

Best Overview Is HBr Polar or Nonpolar? Science Education and Tutorials
Step #1: Calculate the total number of valence electrons. Here, the given molecule is HBrO (or HOBr). In order to draw the lewis structure of HBrO, first of all you have to find the total number of valence electrons present in the HBrO molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).

Estructura De Lewis De Hbr Compuesto
The Lewis structure of HBr contains a single bond between the hydrogen atom and bromine atom. There are three lone pairs on the bromine atom, and the hydrogen atom does not have any lone pair. Contents. Steps #1 Draw skeleton #2 Show chemical bond #3 Mark lone pairs #4 Calculate formal charge and check stability;

How to draw HBr Lewis Structure? Science Education and Tutorials
Hydrogen bromide is an anhydrous gas with no colour having strong irritating smell. It is corrosive in nature and heavier than air. HBr molecule contains one hydrogen atom and one bromine atom in its structure. The molecular weight of HBr is 80.91. HBr has synonyms like bromane, hydrobromic acid, hydrobromide, etc.

Gambarkan struktur Lewis senyawasenyawa berikut!
PROBLEM 3.3.1.4 3.3.1. 4. Methanol, H 3 COH, is used as the fuel in some race cars. Ethanol, C 2 H 5 OH, is used extensively as motor fuel in Brazil. Both methanol and ethanol produce CO 2 and H 2 O when they burn. Write the chemical equations for these combustion reactions using Lewis structures instead of chemical formulas.

HBr Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Techiescientist
Lewis structure of HBrO4 contains the Bromine (Br) atom at the center which is surrounded by three Oxygen atoms (O) and one O-H group. The Bromine atom is double bonded with 3 Oxygen atoms and it is single bonded with O-H group. All the Oxygen atoms have 2 lone pairs. Let's draw and understand this lewis dot structure step by step.

Hbr Lewis Structure Transborder Media
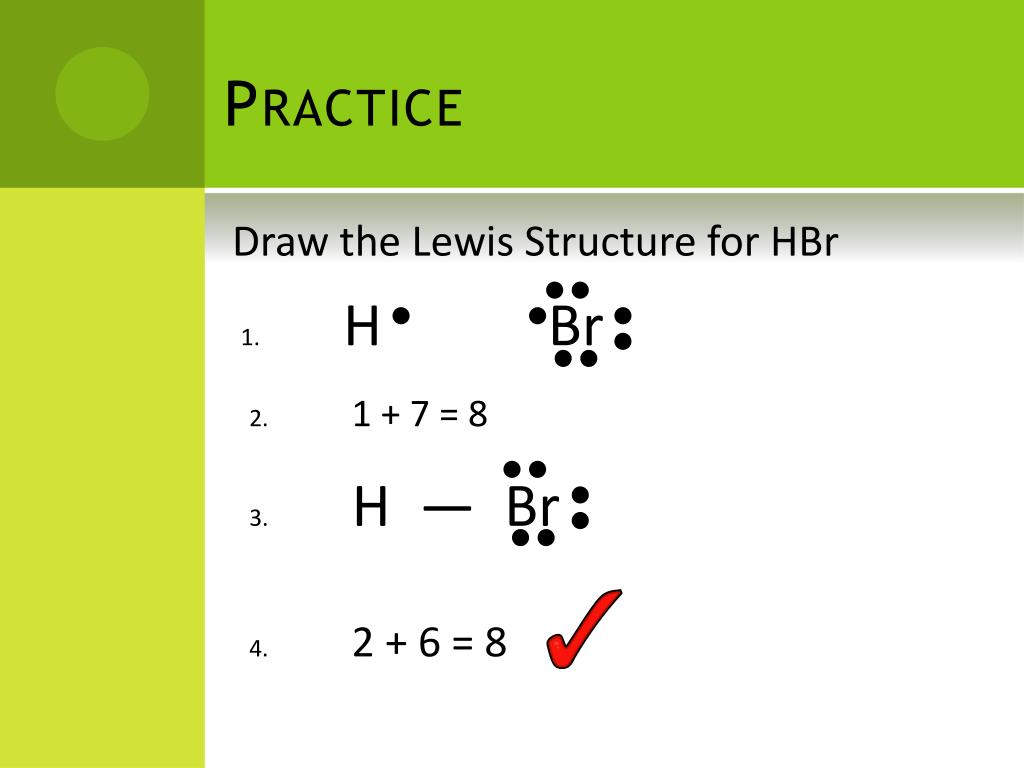
Drawing the Lewis Structure for HBr. HBr is very similar to HF and HCl. Hydrogen has 1 valence electron and Br (in Group 7 with F and Cl) has 7 valence electrons. With the Lewis Structure for HBr remember that Hydrogen only needs 2 valence electrons to have a full outer shell. Be sure that you don't use more than the 8 valence electrons available.

HBr Molecular Geometry Science Education and Tutorials
HBr Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. Hydrogen bromide, HBr is a hydrogen halide compound owing to the fact that bromine belongs to the halogen family. Quite a corrosive and dangerous chemical, it can be useful too in a lot of ways. It is used to prepare a variety of organic and inorganic bromine compounds.

Bromuro de Hidrógeno (HBr) molécula. Fórmula esquelética Fotografía de stock Alamy
Now in the HBr molecule, you have to put the electron pairs between the hydrogen atom (H) and bromine atom (Br). This indicates that the hydrogen (H) atom and bromine (Br) atom are chemically bonded with each other in a HBr molecule. Step 4: Make the outer atoms stable. Place the remaining valence electrons pair on the central atom.

HBr Lewis Structure Lewis Dot Structure for HBrHydrogen Bromide Lewis Structure YouTube
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.

Estructura De Lewis Hbr abstractor
The HBrO (Hypobromous acid) has a bent Lewis structure: a central bromine (Br) atom with 7 valence electrons forms a single bond with a hydrogen (H) atom (1 valence electron) and a single bond with an oxygen (O) atom (6 valence electrons). Oxygen has two lone pairs, resulting in a bond angle slightly less than 109.5°. Total of 14 valence electrons used.