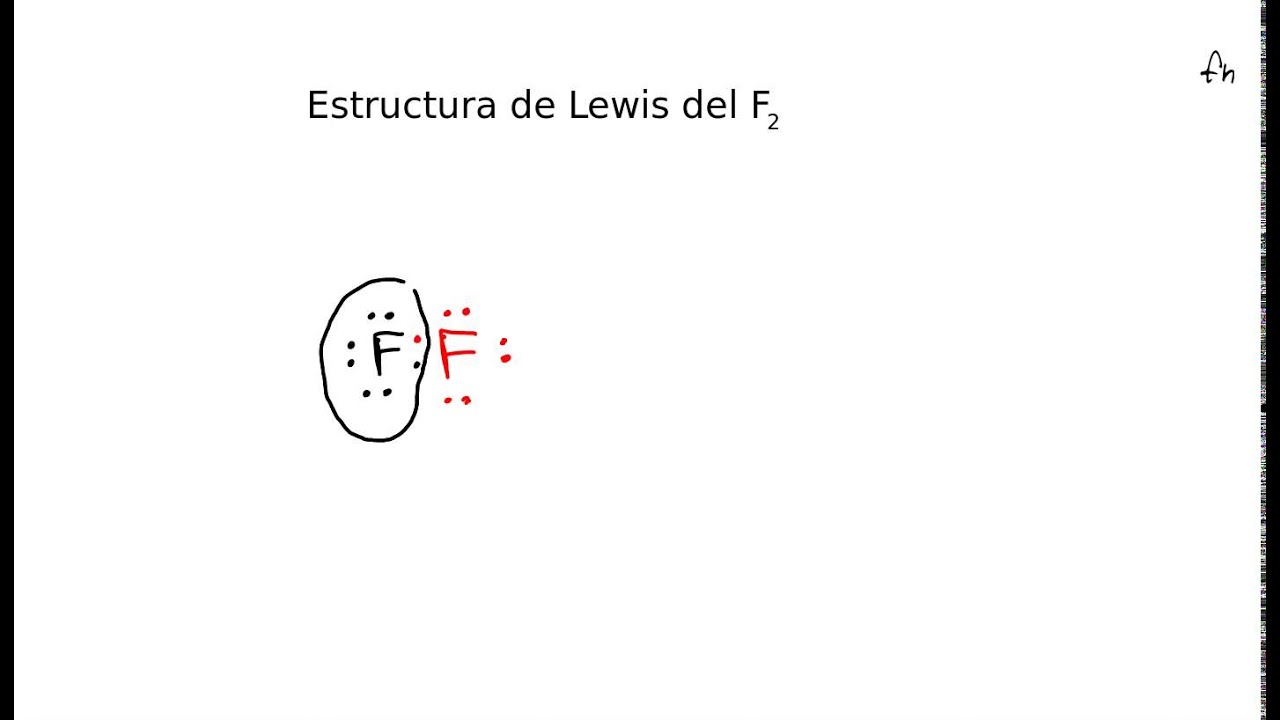
Estructura de Lewis del fluor F2 YouTube
The Octet Rule. The other halogen molecules (F 2, Br 2, I 2, and At 2) form bonds like those in the chlorine molecule: one single bond between atoms and three lone pairs of electrons per atom.This allows each halogen atom to have a noble gas electron configuration. The tendency of main group atoms to form enough bonds to obtain eight valence electrons is known as the octet rule.

Gambarkan struktur Lewis senyawasenyawa berikut!
Fluorine, with the chemical formula F2, is a pale yellow-colored diatomic gas, which has a pungent odor. F2 has a molecular weight of 37.997 g/mol. Its boiling point is −188 °C, and its melting point is −219.67 °C. It is toxic in nature; it can cause chemical burns on the skin and can be lethal if inhaled. It is highly reactive, is.

Cara Mudah Menggambar Struktur Lewis YouTube
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.

Gambarkan Struktur Lewis Nh3
6 Steps to Draw the Lewis Structure of F2 Step #1: Calculate the total number of valence electrons. Here, the given molecule is F2 (Fluorine). In order to draw the lewis structure of F2, first of all you have to find the total number of valence electrons present in the F2 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).

Draw the electron dot structure of F2?
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the.

F2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram Techiescientist
Hi Everyone! In this video, we will help you find out the Lewis Structure of Fluorine gas molecules. It has a very simple structure and with our step-by-step.

F2 Lewis Structure / Is F2 Polar Or Non Polar Fluorine Gas Youtube It has a very simple
Lewis structure of a water molecule. Lewis structures - also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs) - are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as.

F2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram guidetech
A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. The SiF2 molecule contains a total of 4 bond (s). There are 2 non-H bond (s). Images of the chemical structure of SiF2 are given below: The 2D chemical structure image of SiF2 is also called skeletal formula, which is the.

The first step is to put seven valence electrons around the fluorine atom as given in the figure.
The F 2 Lewis structure is similar to Br 2, Cl 2, and I 2 since F, Br, Cl, and I are all in Group 7 and have 7 valence electrons. For the F 2 Lewis structure there are a total of 14 valence electrons available. Transcript: OK, this is Dr. B. We're going to do the Lewis structure for F2, Fluorine gas: a yellow, extremely reactive gas.
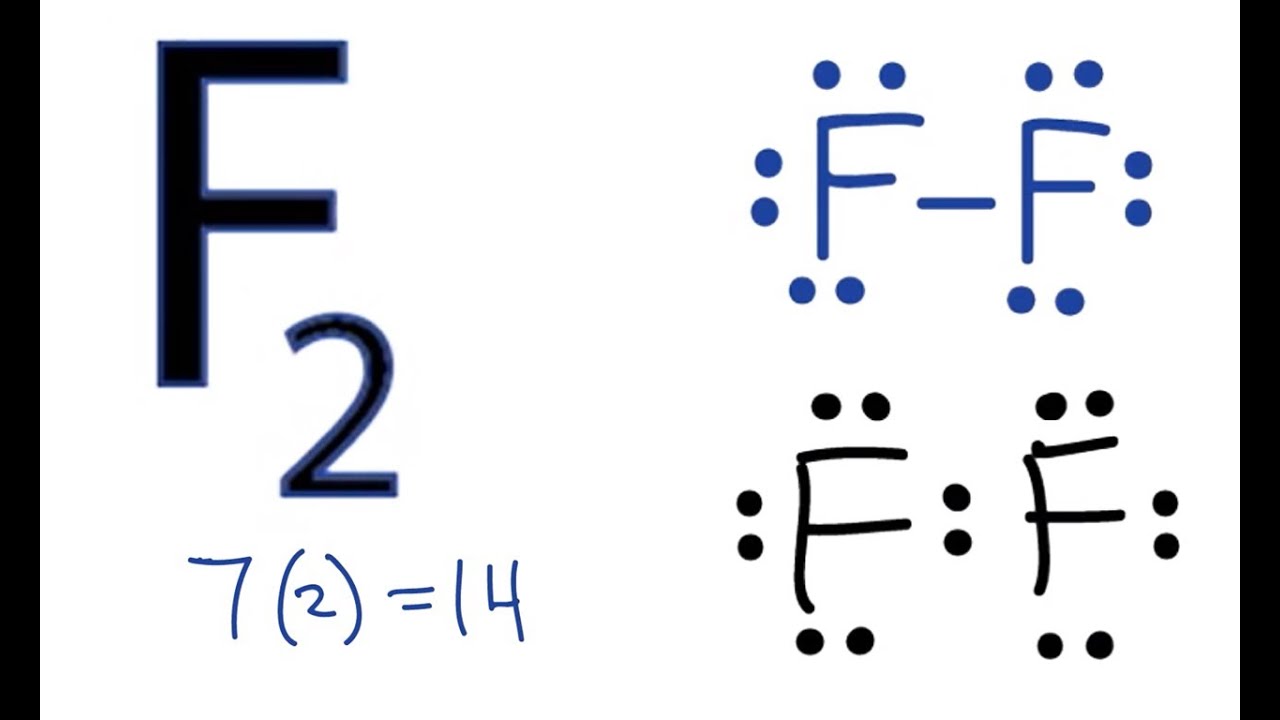
F2 Lewis Structure How to Draw the Lewis Dot Structure for F2 YouTube
F2 is a covalently-bonded molecule, with two F atoms single-bonded to each other. There are three lone pairs on each of the F atoms.Check me out: http://www..
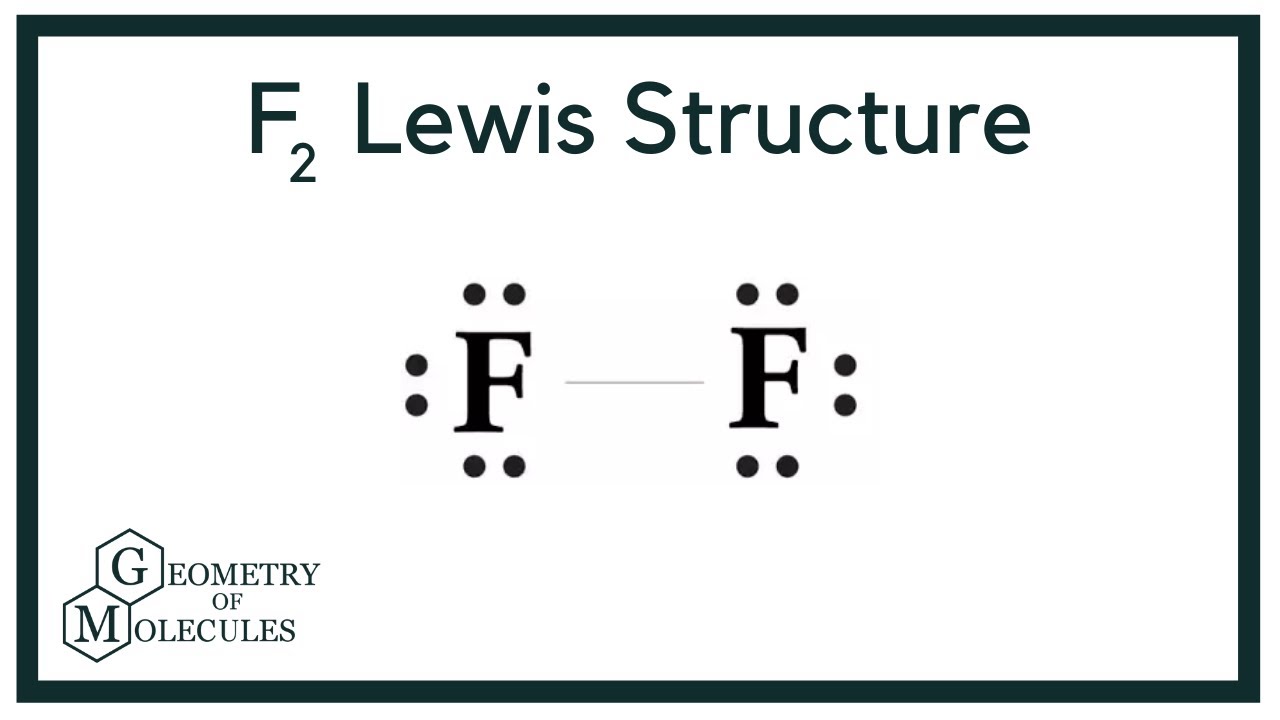
F2 Lewis Structure (Fluorine Gas) YouTube
A step-by-step explanation of how to draw the F2 Lewis Dot Structure (Diatomic Fluorine).Note that Diatomic Fluorine is often called Molecular Fluorine or ju.
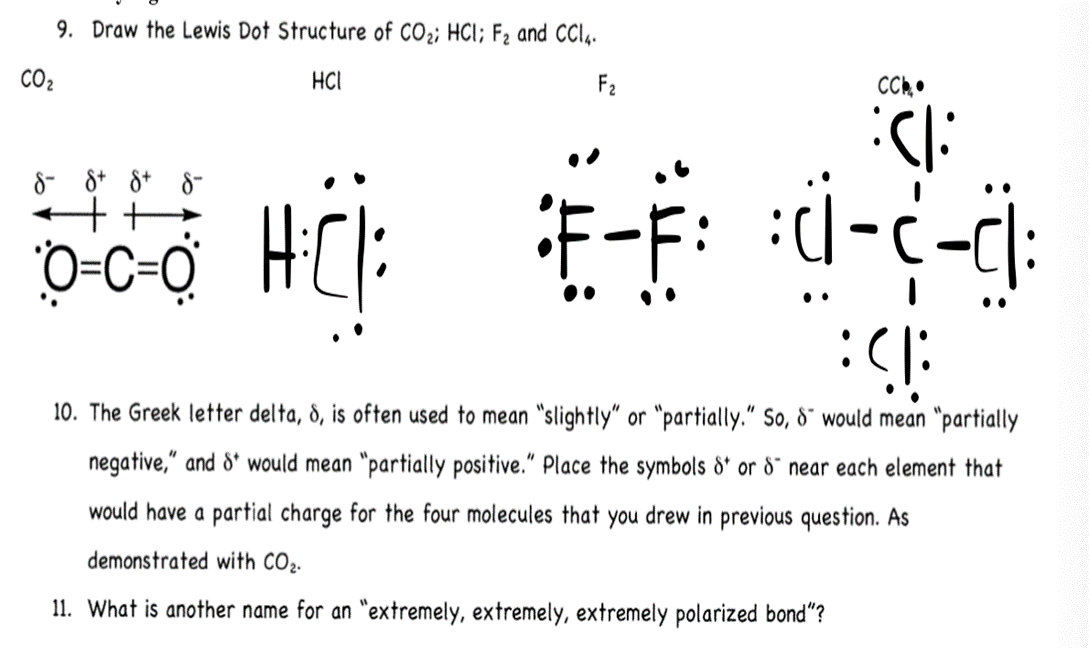
(Get Answer) 9. Draw The Lewis Dot Structure Of CO2; HCI; F2 And CCIA. CO2 HCI... Transtutors
Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

So far, we’ve used 8 of the F2 Lewis structure’s total 14 outermost valence shell electrons
Step 3: Connect each atoms by putting an electron pair between them. Now in the F2 molecule, you have to put the electron pairs between both the fluorine atoms (F). This indicates that both the fluorine (F) atoms are chemically bonded with each other in a F2 molecule. Step 4: Make the outer atoms stable. Place the remaining valence electrons.

F2 Lewis Structure
A step-by-step explanation of how to draw the F2 Lewis Dot Structure (Fluorine gas).For the F2 structure use the periodic table to find the total number of v.

Perhatikan gambar struktur Lewis beberapa senyawa berikut...
F2 Lewis Structure A. Definition and concept. The Lewis structure is a representation of a molecule's valence electrons using dots and lines to indicate bonds. For the F2 molecule, there are two fluorine atoms bonded together through a single covalent bond. Each fluorine atom has 7 valence electrons, and they share one electron pair to form.

Draw the electron dot structure for F2.
Steps. To properly draw the F 2 Lewis structure, follow these steps: #1 Draw a rough sketch of the structure. #2 Next, indicate lone pairs on the atoms. #3 Indicate formal charges on the atoms, if necessary. Let's break down each step in more detail.