
What Is Delta Tf in Chemistry
3 Answers. Sorted by: 3. Certainly, you agree dH =CPdT d H = C P d T if we're at constant pressure. If H H and CP C P don't actually depend on pressure, then you can use this equation regardless of whether pressure changes. However, to determine CP C P and H H you first need an equation of state (such as PV = NkT P V = N k T ).

Rumus Delta S Kimia Bit CDN
The mass flow rate m [kg/s] is a measurement of the amount of water flowing around the hot water loop.. The specific heat capacity Cp [kJ/kg/°C] is a thermodynamic property specific of the fluid used to transfer heat. We could manipulate the specific heat capacity only by changing the fluid used in the loop. Water is a good fluid choice for cost and safety considerations.
Using Y to Delta Transformation to Find Currents
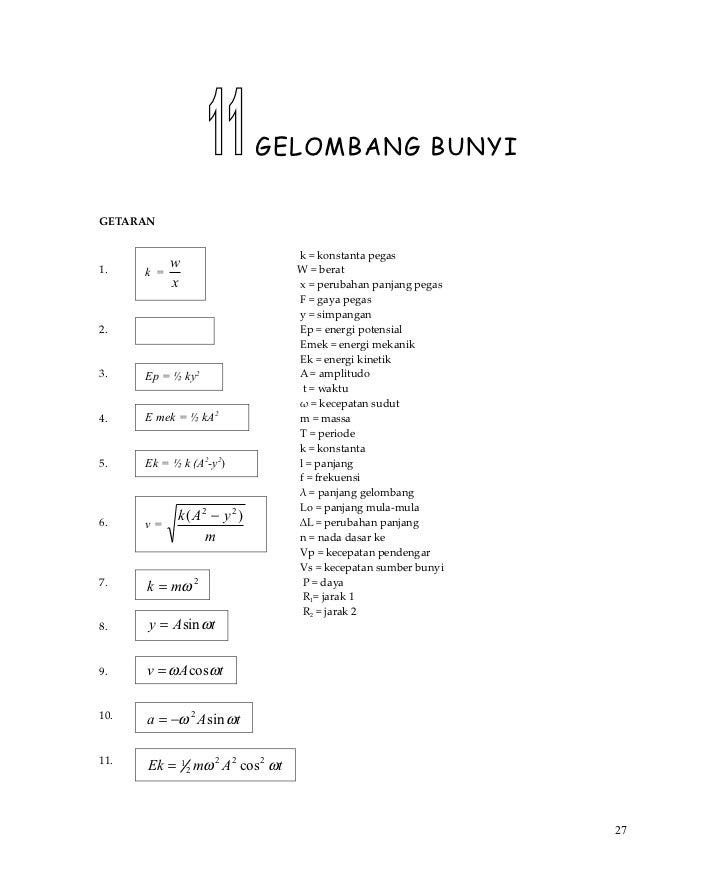
ΔTf = Tf pelarut murni −Tf larutan. Rumus di atas berlaku untuk larutan nonelektrolit. b. ΔTb tidak berlaku untuk larutan yang mudah menguap.. Misalkan = delta yaa Tb = m . Kb Tb/ m = kb Dengan: Tb = kenaikan titik didih m = molalitas kb = tetapan kenaikan titik didih #cmiiw 27 November 2020 pukul 00.32

√ Rumus Delta T
Transfer functions are a frequency-domain representation of linear time-invariant systems. For instance, consider a continuous-time SISO dynamic system represented by the transfer function sys(s) = N(s)/D(s), where s = jw and N(s) and D(s) are called the numerator and denominator polynomials, respectively. The tf model object can represent SISO or MIMO transfer functions in continuous time or.

Termokimia Pengertian Persaman Reaksi Rumus Dan Contoh Soal Soal Sexiz Pix
Temperature (T) = 80.0 K. Specific heat (c) = 1676 KJ. Now we have to convert the specific heat into Joules because it is in Kilojoules. So, the conversion is like this. 1 KJ = 1,000 J. So, 1676 KJ = 1,000 × 1676 = 16,76,000 J. Now put all the values in the formula. But, before that, we have to reorganize the formula to find specific heat.

√ Rumus Delta T
Delta T Formula. The following formula is used to calculate delta T. ΔT = T2 - T1 ΔT = T 2 − T 1. Where ΔT is the change in temperature (delta T) T2 is the final temperature. T1 is the initial temperature. To calculate delta T, simply subtract the initial temperature from the final temperature.

√ Rumus Delta T
jumlah nilai NaCl agar isotonis pada sediaan 5 mL = (0,9/100) x 5 mL = 0,045 gram. Sedangkan jumlah nilai NaCl dalam sediaan (berdasarkan resep) yaitu. Rumus E x W. Ampisilin Na = 0,1 gr x 0,16 = 0,016. Isoniazid = 0,05 gr x 0,25 = 0,0125. jadi total nilai kesetaraan NaCL dalam sediaan = 0,016 + 0,0125 = 0,0285 gram. Sehingga agar Isotonis :
√ Rumus Delta T
Freezing point depression is a colligative property observed in solutions that results from the introduction of solute molecules to a solvent. The freezing points of solutions are all lower than that of the pure solvent and is directly proportional to the molality of the solute. ΔTf = Tf(solvent) −Tf(solution) = Kf × m Δ T f = T f ( s o l.
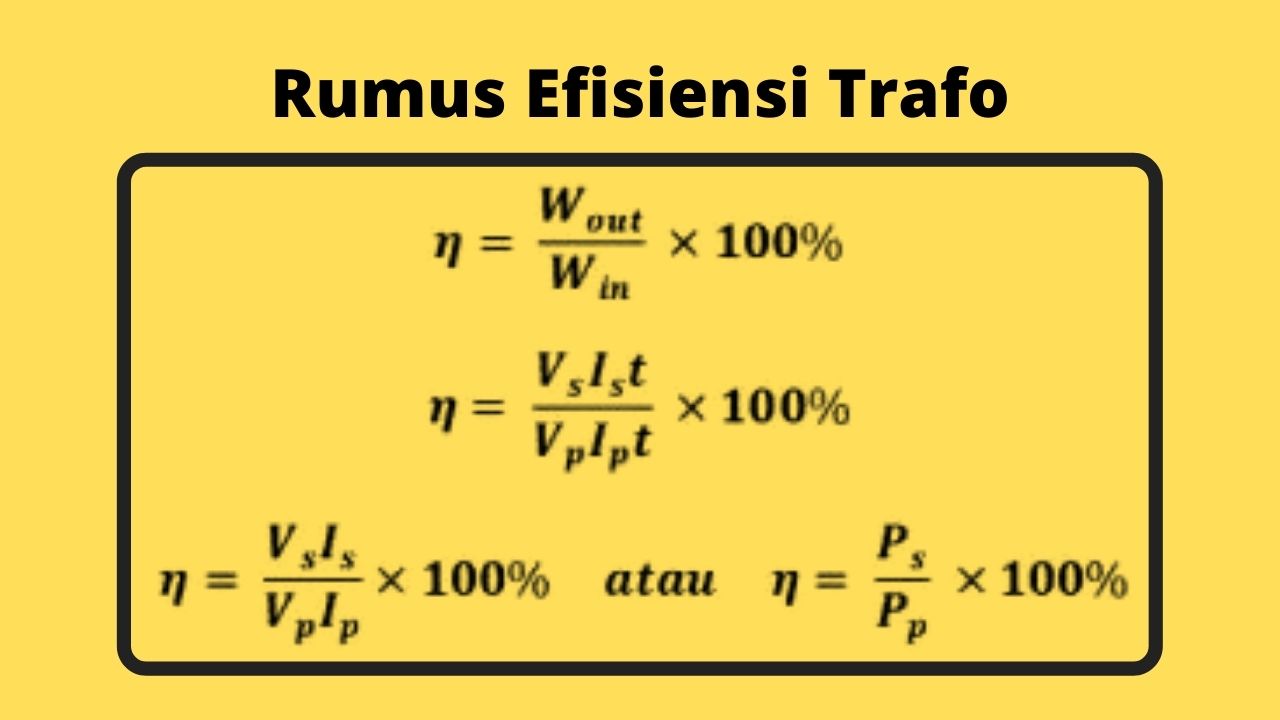
√ Transformator Pengertian, Fungsi, Cara Kerja, Jenis Rumus
AP Chemistry Johns Hopkins University Center For Talented Youth Lab Information Freezing Point Depression Calculations ΔT f = iK f m ΔT

What Is Delta Tf in Chemistry
Step 2: Subtract the Initial Temperature from Final Temperature. Calculating delta T simply involves subtracting the initial temperature from the final temperature. The formula for this calculation is straightforward: Delta T = Tf - Ti. Note that if the final temperature is lower than the initial temperature, delta T will be a negative number.

Transformation of Resistances (Star to Delta and Delta to Star) Brilliant Math & Science Wiki
بِسْــــــــــــــــــمِ اللهِ الرَّحْمَنِ الرَّحِيْمِ. Assalamualaikum w.r.b. teman-teman. Slamat datang dibisakimia.com insyaallah pasti bisa. Berikut ialah Rumus Serta Soal dan Pembahasan SIFAT KOLIGATIF LARUTAN. Jadi ini adalah bab I mapel kimia dikelas XII .

Formula Description Heat Delta Temperature ภาพประกอบสต็อก 1015197763 Shutterstock
Specific Heat Formula. When heat energy is added to a substance, the temperature will change by a certain amount. The relationship between heat energy and temperature is different for every material, and the specific heat is a value that describes how they relate. heat energy = (mass of substance) (specific heat) (change in temperature) Q = mc∆T.

What Is Delta Tf in Chemistry
F = m * delta p / delta t, where delta t is the 1 second the ball is in contact with the wall during the 'bounce' and delta p is the same as above: 2v. We get F = m * 2v / 1 = 2*mv. Clearly the method shown in the video gives a much smaller force than when considering time as only the time when the object is applying the force to the wall.

Pelajaran Soal Rumus Kenaikan Titik Didih Penurunan Titik Beku My XXX Hot Girl
Rumus: idf (t) = log (N / (df + 1)) tf-idf now adalah ukuran yang tepat untuk mengevaluasi seberapa penting sebuah kata bagi sebuah dokumen dalam collection atau corpus. ada banyak variasi TF-IDF yang berbeda, tetapi untuk saat ini mari kita berkonsentrasi pada versi dasar ini. Rumus: tf-idf (t, d) = tf (t, d) * log (N / (df + 1))

What Is Delta Tf in Chemistry
Berikut penjelasan kelompok sifat koligatif: 1. Koligatif Larutan - Penurunan tekanan uap jenuh (Δ Tp ) Penurunan tekanan uap jenuh adalah selisih tekanan uap pelarut murni dan tekanan uap larutan . tabel penurunan tekanan uap jenuh larutan non elektrolit dan elektrolit ↓. Uraian. Larutah non elektrolit.

√ Rumus Delta T
Step 1: Calculate the freezing point depression of benzene. Tf = (Freezing point of pure solvent) - (Freezing point of solution) Step 2 : Calculate the molal concentration of the solution. molality = moles of solute / kg of solvent. Step 3: Calculate Kf of the solution. Tf = (Kf) (m)