
Jual Reagen Molisch Larutan Molisch Molisch Reagent 100 mL di Lapak Rofa Yulia Azhar Bukalapak
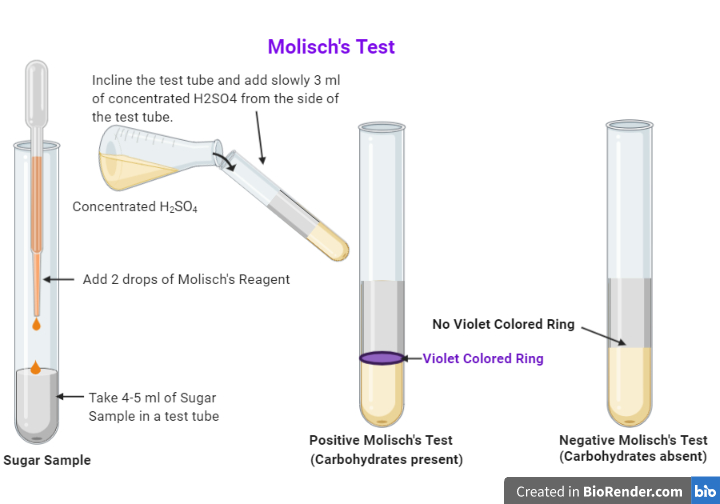
Procedure. Add 1-2 ml of unknown solution in a test tube. Add 2-3 drops of Molisch's reagent ad mix well. Hold the test tube inclined and carefully add 1-2 ml of Conc. Sulfuric acid down the side of the tube to make a layer of it at the bottom of the tube.

Nice M31771 Molisch Reagent, 125ml, For Laboratory, Packaging Type Bottle at Rs 131/125 ml in
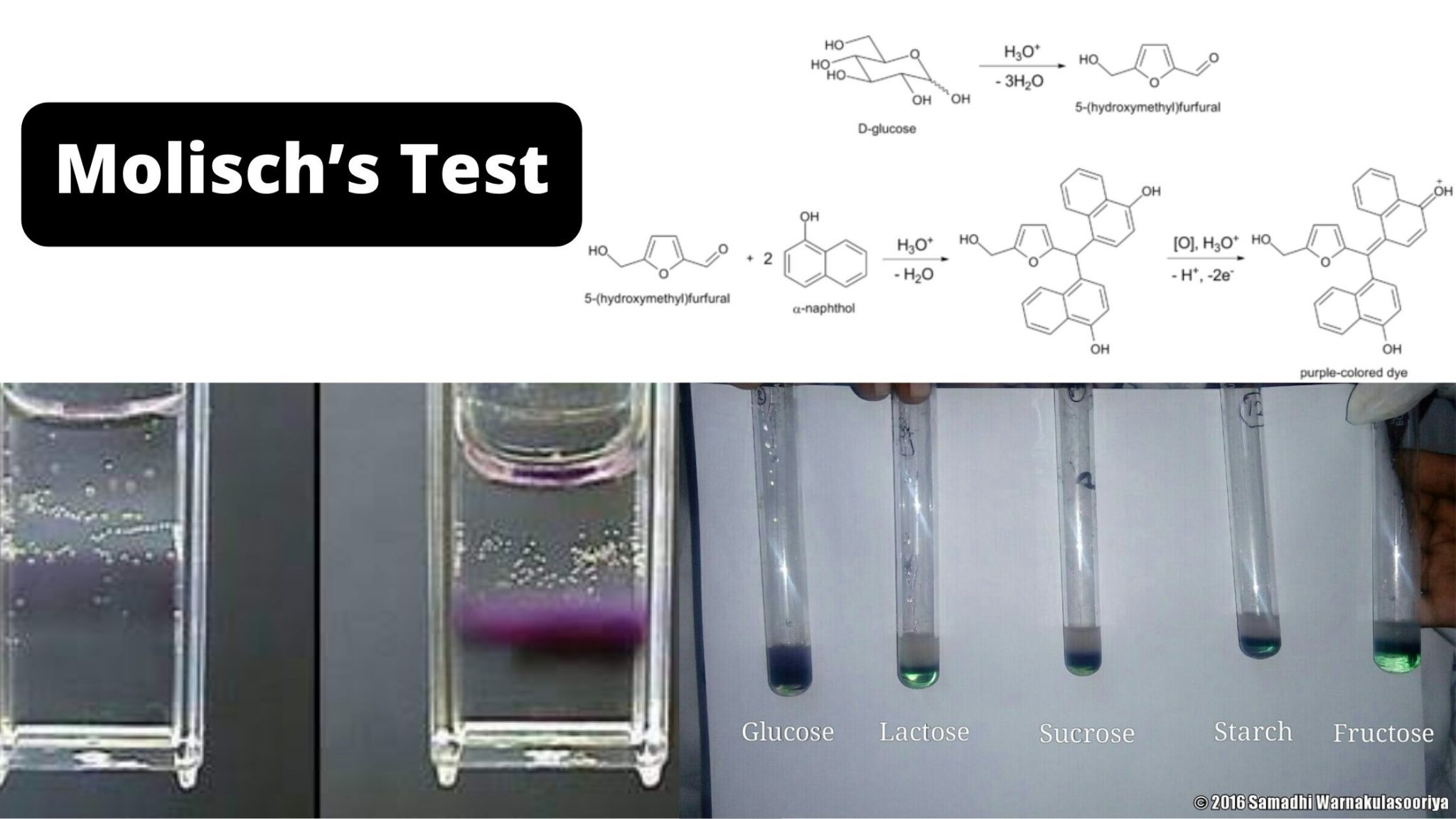
Wikipedia says. Molisch's test is a sensitive chemical test, named after Austrian botanist Hans Molisch, for the presence of carbohydrates, based on the dehydration of the carbohydrate by sulfuric acid or hydrochloric acid to produce an aldehyde, which condenses with two molecules of a phenol (usually α-naphthol, though other phenols such as resorcinol and thymol) also give colored products.

Réaction de Molisch
Molisch test is a chemical test to detect carbohydrates (monosaccharide, disaccharide, and polysaccharide) and glycoprotein in an analyte. It can be used to differentiate proteins and amino acids from carbohydrates. The test has been named after Czech-Austrian botanist Hans Molisch [1,2]. Principle of Molisch's Test
Molisch’s Test Objectives, Principle, Reagents, Procedure and Result Online Biology Notes
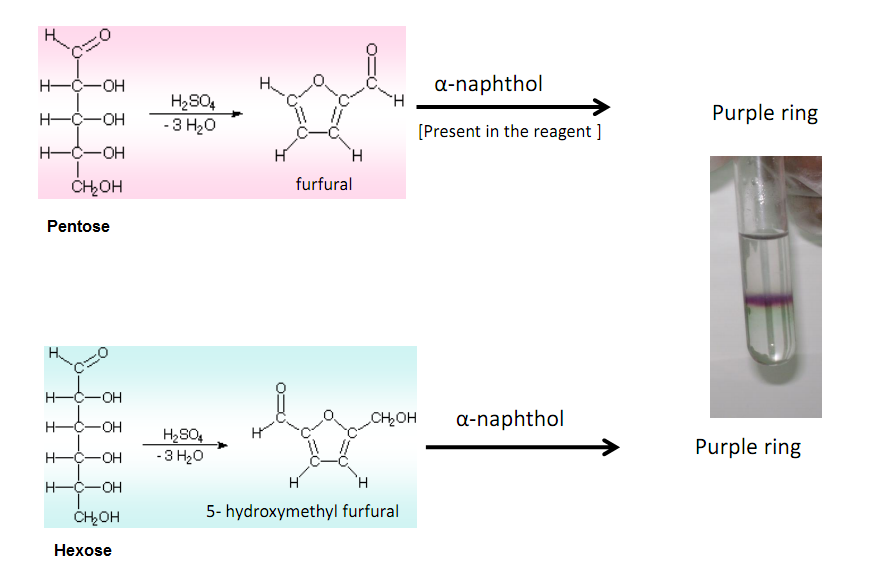
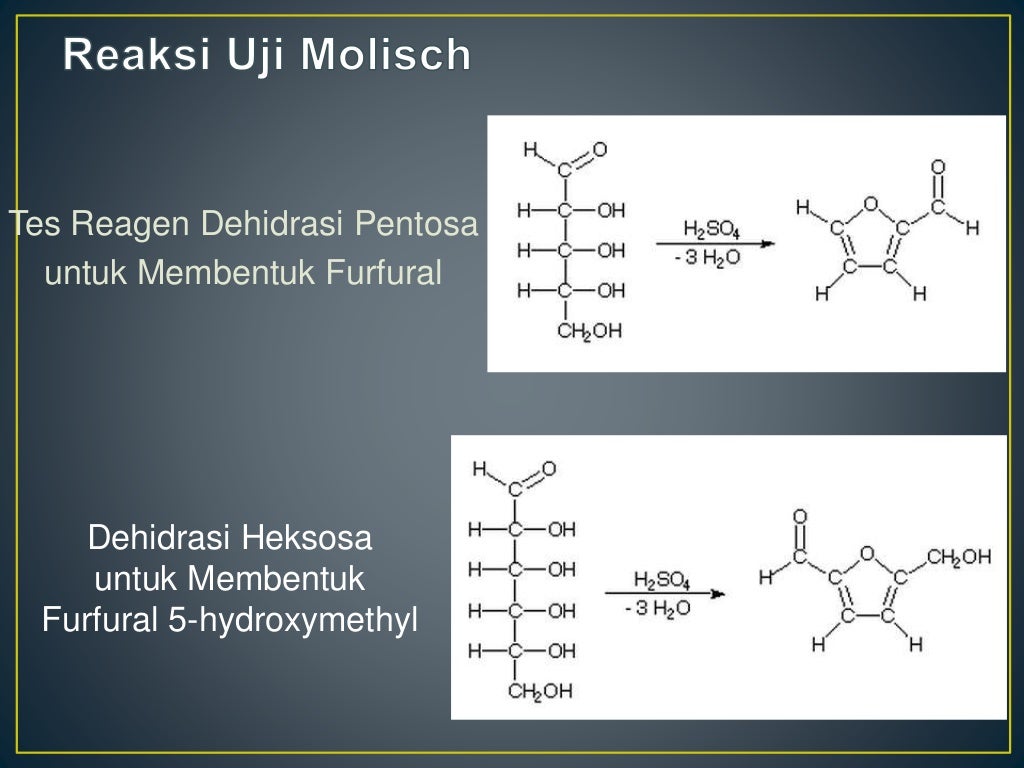
Reaksi Uji Molisch Monosakarida disakarida dan polisakarida (kecuali triosa dan tetrosa) akan menghasilkan reaksi positif, dan glikoprotein serta asam nukleat memiliki reaksi positif karena semua senyawa ini dihidrolisis menjadi monosakarida dengan asam mineral kuat.

Uji molisch
Molisch's test is a chemical test that detects the presence of carbohydrates in an analyte. This test is named after Czech-Austrian botanist Hans Molisch, who discovered it. Q2 What is the principle of Molisch's Test? Molisch's test is based on the dehydration of sulphuric acid into furfural.
Uji molisch
Molisch test is not a specific test for carbohydrates. Furfurals as such or furfural yielding substance, some organic acids like citric acids, lactic acid, oxalic acid, formic acid, etc. can give a positive result. References and Sources. Tiwari A. (2015). Practical Biochemistry. LAP Lambert Academic Publishing.

Molisch's Test Practical Experiment YouTube
Product name : Molisch's Reagent Product Code : 866320 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses : Laboratorychemicals, Industrial & for professional use only. 1.3 Details of the supplier of the safety data sheet Company

Reaksi Uji Karbohidrat dengan Uji Molisch A Learner
THE USE OF THE MOLISCH (a-NAPHTHOL) REACTIONS IN THE STUDY OF SUGARS IN BIOLOGICAL FLUIDS BY JOHN H. FOULGER (From the Department of Pharmacology, Medicine, University Cincinnati) of Cincinnati, College of (Received for publication, April 16, 1931) The cy-naphthol (Molisch) reaction for sugars has been neglected as a possible basis for a quantit.

Promo Reagen Molisch/Pereaksi Molisch/Larutan Molisch For Analisis 100 Ml Diskon 23 di Seller
Molisch's test works on a simple principle which is explained elaborately in the next heading. Principle of Molisch's Test: To detect the presence of carbohydrates, the solution is first treated with a strong acid.This is for hydrolyzing the carbohydrate to monosaccharide. A compound named furfurol is then made when water is removed from.
Molisch's Test Definition, Principle, Procedure, Result, Uses
Kemudian, ditambahkan 2 tetes reagen Molisch dan dihomogenkan (dikocok hingga merata). Selanjutnya, tabung reaksi tersebut dimiringkan d an ditambahkan H 2 SO 4 pekat melalui dinding tabung .

Reagente de Molisch Teste para indicar a presença de Carboidratos Engquimicasantossp
Reagen molisch (0,5 gr alfa-naftol dalam 20 ml etanol 96%) H 2 SO 4 (Asam sulfat) pekat Bahan yang akan diuji Langkah kerja: Masukkan 2 ml bahan yang akan diuji ke dalam tabung reaksi. Tetesi dengan 2 tetes reagen molisch Miringkan tabung reaksi dan tuang 2 ml asam sulfat pekat melalui dinding tabung (jangan dikocok).

Liquid Molisch Reagent, Chemical Grade, For Laboratory, Rs 190 /unit ID 4205568191
Procedure The test solution is combined with a small amount of Molisch's reagent ( α-naphthol dissolved in ethanol) in a test tube. After mixing, a small amount of concentrated sulfuric acid is slowly added down the sides of the sloping test-tube, without mixing, to form a layer.

Molisch’s Test Objectives, Principle, Reagents, Procedure and Result Online Biology Notes
Molisch Test is a qualitative analysis used to identify the existence of carbohydrates in a given sample. Czech-Austrian botanist Hans Molisch discovered this test. , He added a solution of α-naphthol in ethanol to the analyte and then included a few drops of concentrated Sulphuric acid to the mixture. As a result, a purple or a purplish-red ring was generated at the surface of contact.
Molisch’s Test Objective, Principle, Procedure, Result
Product name : Molisch Reagent Manufacturer/Supplier Trade name: Manufacturer/Supplier Article number: S25754 Recommended uses of the product and uses restrictions on use: Manufacturer Details: AquaPhoenix Scientific 9 Barnhart Drive, Hanover, PA 17331 Supplier Details: Fisher Science Education 15 Jet View Drive, Rochester, NY 14624

Pendidikan/ Kesehatan LAPORAN PRAKTIKUM UJI MOLISCH BIOKIMIA
1.Molisch's Reagent: i- α-naphthol, ii- Ethyl alcohol. 2. Concentrated H2SO4. 3. Original solution (O.S)- containing a carbohydrate. PROCEDURE: To 2ml of sugar solution (original solution) add 2 to 3 drops of Molisch's reagent. Mix thoroughly. Carefully pour 5 ml concentrated H2SO4 along the side of the test tube.

Molisch’s Test Objectives, Principle, Reagents, Procedure and Result Online Biology Notes
Procedure of Molisch's test: Take 2ml of sample in dry test tube. Take 2ml of distilled water in another tube as control. Add 2-3 drops of Molisch's reagent to the solution. Gently pipette 1ml conc. H2SO4 along the side of the tube so that two distinct layers are formed. Observe color change at the junction of two layers.