Atomic Theory IV Chemistry Visionlearning
The orbital occupied by the hydrogen electron is called a 1s orbital. The "1" represents the fact that the orbital is in the energy level closest to the nucleus. The "s" tells you about the shape of the orbital. s orbitals are spherically symmetric around the nucleus - in each case, like a hollow ball made of rather chunky material with the.

À quoi correspondent les orbitales électronique ? Et les nombres quantiques ? CouleurScience
Mechanistic insights into electrocatalytically reduced OER performance in marigold-like trimetallic NiFe-based LDH: Charge localisation and d-band orbital filling S. Deka, M. K. Jaiswal, P. Rajput and B. Choudhury, J. Mater. Chem. A, 2024, Accepted Manuscript , DOI: 10.1039/D3TA07489G

3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts
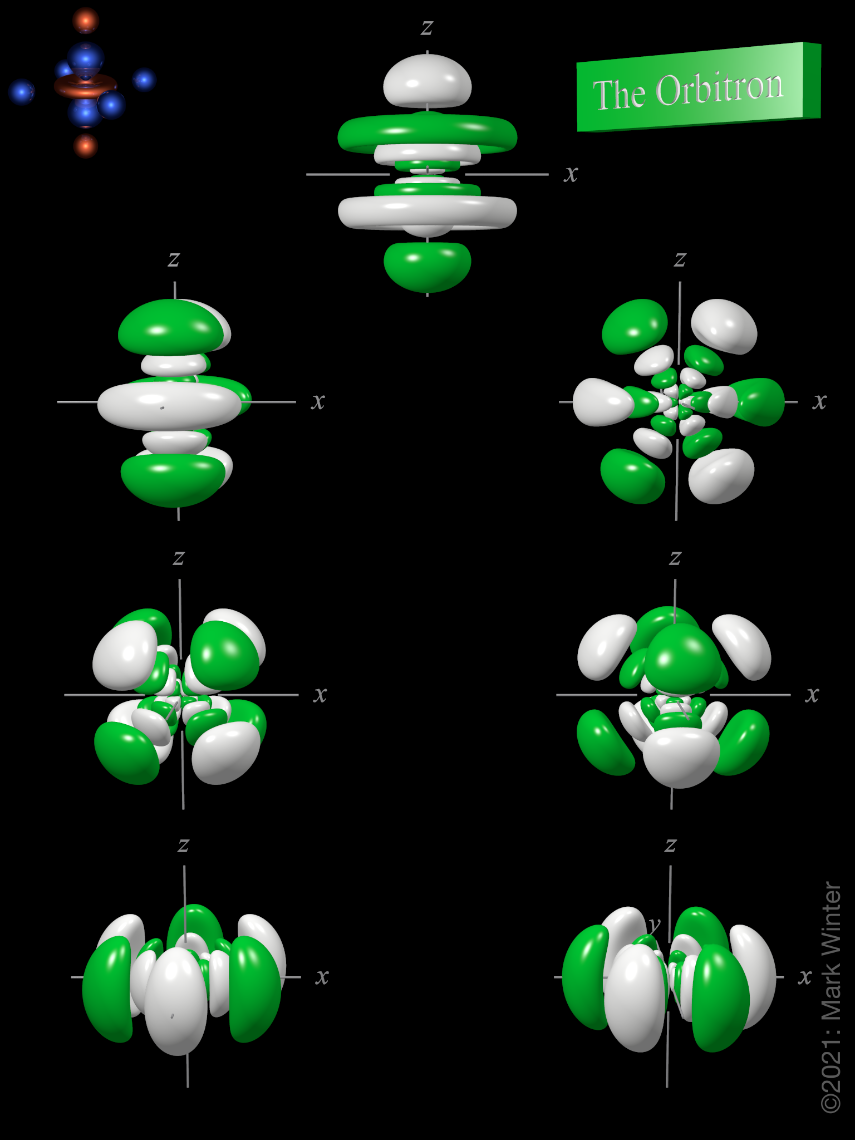
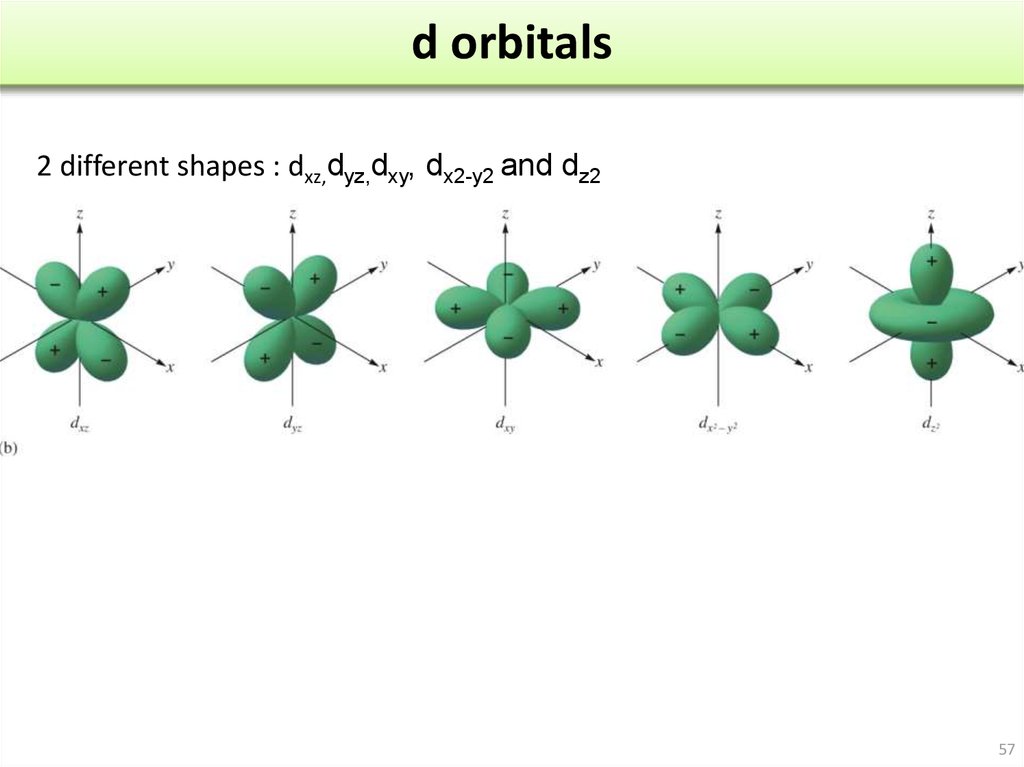
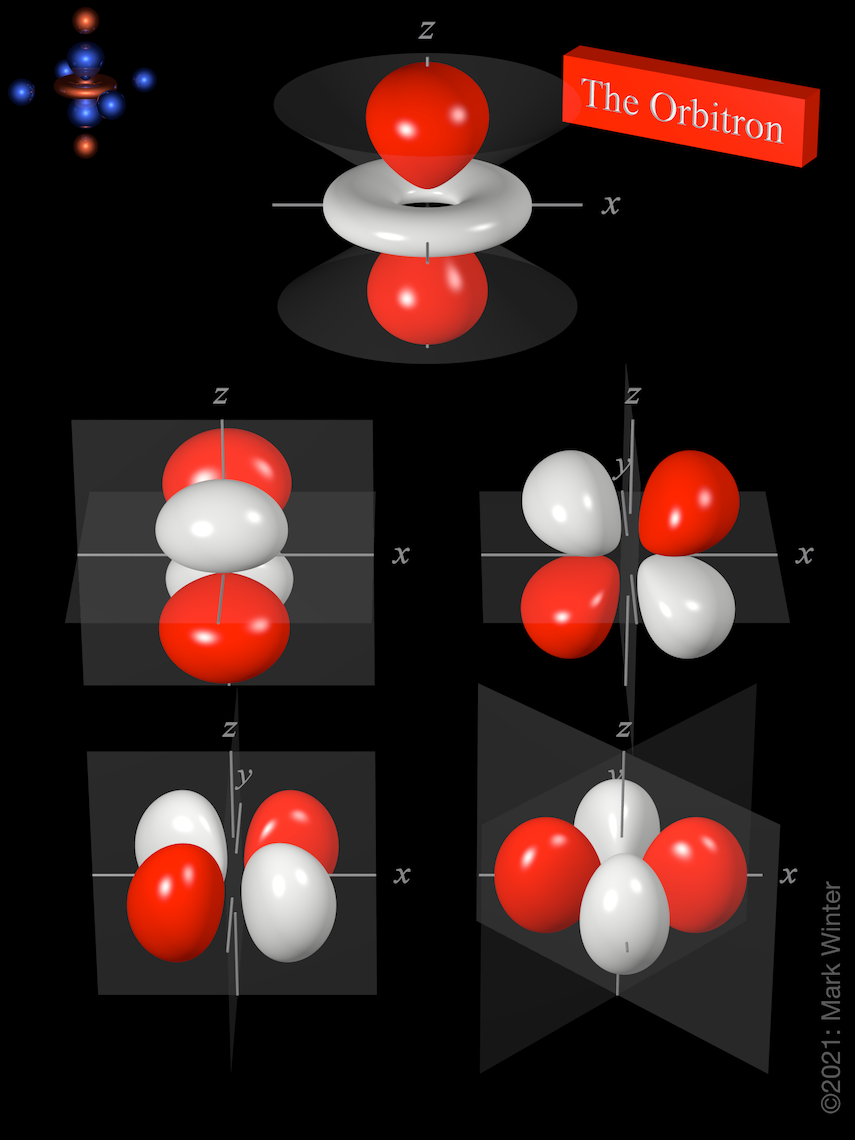
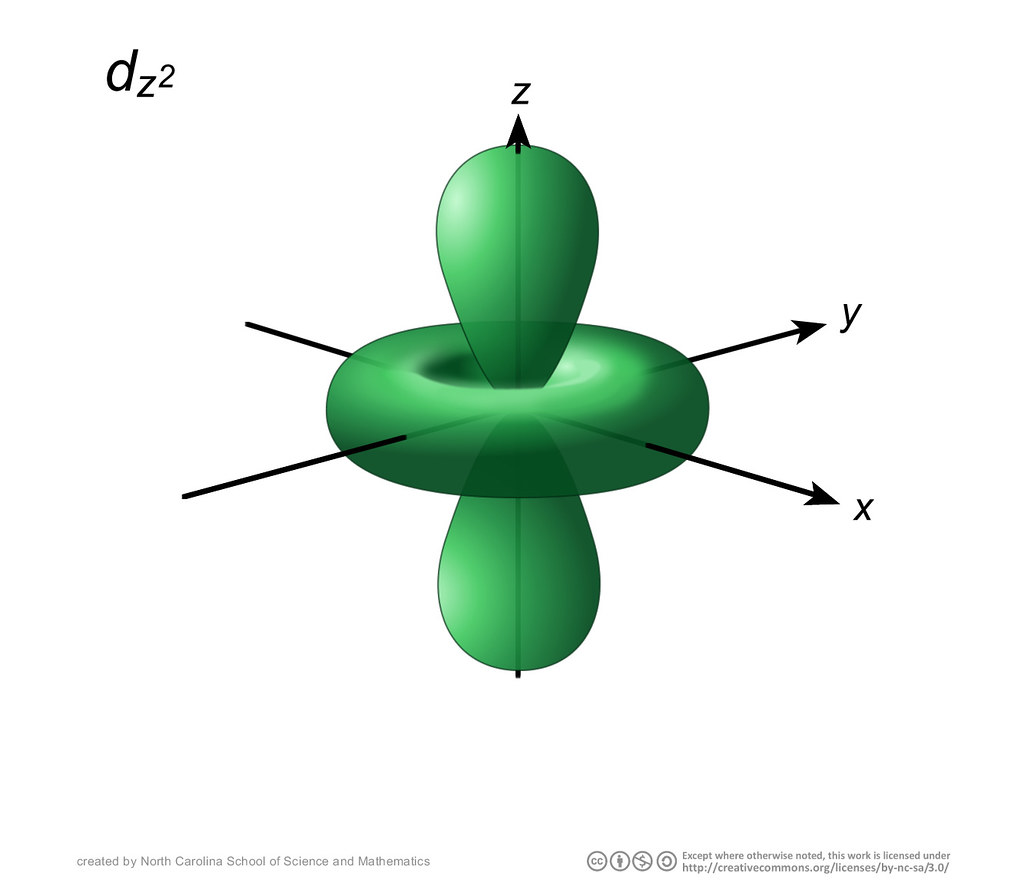
A fourth d orbital has lobes lying along the x and y axes; this is the \(3d_{x^2−y^2}\) orbital. The fifth 3d orbital, called the \(3d_{z^2}\) orbital, has a unique shape: it looks like a \(2p_z\) orbital combined with an additional doughnut of electron probability lying in the xy plane. Despite its peculiar shape, the \(3d_{z^2}\) orbital is.
Atomic Orbitals D
Generally, there are three types of bonding and antibonding interactions that may occur with d d orbitals: sigma ( σ σ ), pi ( π π ), and delta ( δ δ) bonds. Figure 5.1.3.1 5.1.3. 1: The five 3d 3 d orbitals are shown. The orientation of the axes is consistent and the z z axis is horizontal for convenience in drawing bonding along the z z.

Shapes of Orbitals and their Types Chemistry Skills
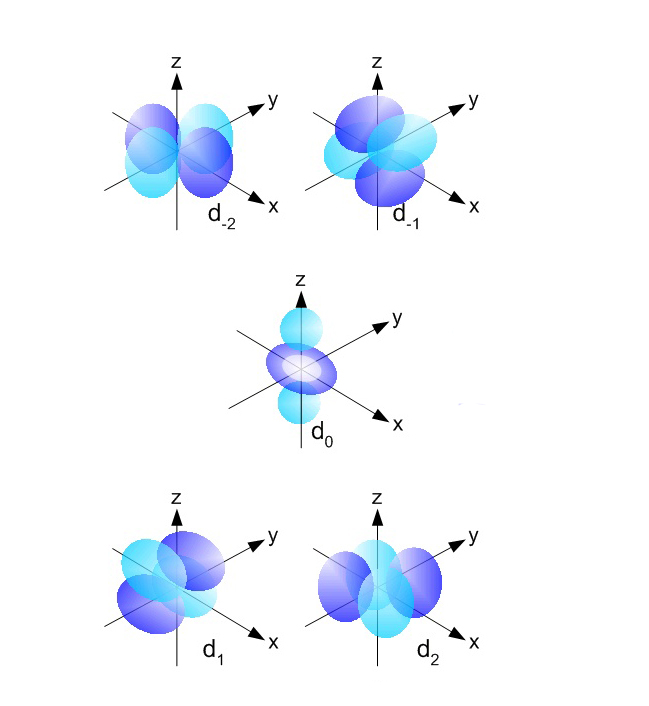
configuration species excited states d^0 Ca+2, Ti+4, Sc+3 0 d^1 Ti+3 1 d^2 V+3 3 d^3 Cr+3 3 d^4 Mn+3 1^*d^5 Mn+2, Fe+3 0 d^6 Fe+2 1^*d^7 Co+2 3 d^8 Ni+2 3 d^9 Cu+2 1 d^{10} Zn+2 0^* *Jahn-Teller distortion breaks the expected degeneracy. See also: Electron Orbital , Transition Element . Quantum Chemistry: Orbitals: d-Orbitals : configuration:
Atomic structure and properties. (Chapter 3) презентация онлайн
An orbital is the quantum mechanical refinement of Bohr's orbit. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived regions of space with different probabilities of containing an electron.

Atom Electrons, Orbitals, Energy Britannica
In quantum mechanics, an atomic orbital ( / ˈɔːrbɪtəl /) is a function describing the location and wave-like behavior of an electron in an atom. [1] This function describes the electron's charge distribution around the atom's nucleus, and can be used to calculate the probability of finding an electron in a specific region around the nucleus. [2]
D Orbitals Signs
The Shape of d Orbitals Solved Example Frequently Asked Questions - FAQs Orbitals Chemistry There are four different kinds of orbitals, denoted s, p, d and f each with a different shape. Of the four, s and p orbitals are considered because these orbitals are the most common in organic and biological chemistry.
How Many Different D Orbitals Are Within the 3d Sublevel ChrishasDavidson
digedag 10 years ago An orbital is a space where a specific pair of electrons can be found. We classified the different Orbital into shells and sub shells to distinguish them more easily. This is also due to the history when they were discovered. Start with the easy. Imagine shells around the nucleus, that get bigger and bigger.

Atomic Orbitals D
d Atomic Orbitals

6.6 3D Representation of Orbitals Chemistry LibreTexts
As the world's leading provider of launch services - and the only provider with an orbital class reusable rocket - SpaceX has deep experience with both spacecraft and on-orbit operations. Learn More. 30 DAY TRIAL. If not satisfied, return Starlink for a full refund. Service Address.

Five D Orbitals
Orbital-free density functional theory (OFDFT) is a quantum chemistry formulation that has a lower cost scaling than the prevailing Kohn-Sham DFT, which is increasingly desired for contemporary.

Atomic Orbitals D
Electronic Structure of Atoms s, p, d, f Atomic Orbitals In today's post, we will talk about the atomic orbitals. So, first, what is an orbital? In a formal, quantum mechanical definition, orbitals are essentially probability distribution maps for electrons within atoms.

FileD orbitals.svg Wikipedia, the free encyclopedia
orbital, in chemistry and physics, a mathematical expression, called a wave function, that describes properties characteristic of no more than two electrons in the vicinity of an atomic nucleus or of a system of nuclei as in a molecule. An orbital often is depicted as a three-dimensional region within which there is a 95 percent probability of.
Atomic Orbitals D
There are a total of five d orbitals and each orbital can hold two electrons. The transition metal series is defined by the progressive filling of the 3d orbitals.These five orbitals have the following ml values: ml=0, ±1, ±2, Explore other atomic orbitals s-orbitals | 2p-orbitals | 3p-orbitals | 3d-orbitals | 4f-orbitals
dorbital_dz2.jpg NCSSM, a publicly funded high school in … Flickr
The d-orbitals are even less well known than the s and p-orbitals which preceded them. Since they are inti-mately involved in transition metal chemistry, chemists must understand them reasonably well. We deal with them here, starting with the d z2 orbital, and then con-sidering the d xy orbital and the d x 2−y orbital. III. d z2 ORBITALS