
No atom sign on white background. premium vector in Adobe Illustrator ai ( .ai ) format
Calcium (20 Ca) has 26 known isotopes, ranging from 35 Ca to 60 Ca. There are five stable isotopes (40 Ca, 42 Ca, 43 Ca, 44 Ca and 46 Ca), plus one isotope (48 Ca) with such a long half-life that for all practical purposes it can be considered stable. The most abundant isotope, 40 Ca, as well as the rare 46 Ca, are theoretically unstable on energetic grounds, but their decay has not been observed.

"no atom" Stockfotos und lizenzfreie Bilder auf Bild 74342139
calcium (Ca), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. It is the most abundant metallic element in the human body and the fifth most abundant element in Earth 's crust. Element Properties. atomic number. 20.
Periodic Table Carbon Atomic Number Periodic Table Timeline
46 Ca Calcium-46 is a stable isotope containing 26 neutrons. 0.004% of natural calcium is calcium-46. As with calcium-40, the internal structure of the calcium-46 atom is theoretically unstable and could be radioactive. No one has ever observed the decay of a calcium-46 atom. 48 Ca Calcium-48 is almost a stable isotope containing 28 neutrons.

182 Calcium Atomic Properties Images, Stock Photos & Vectors Shutterstock
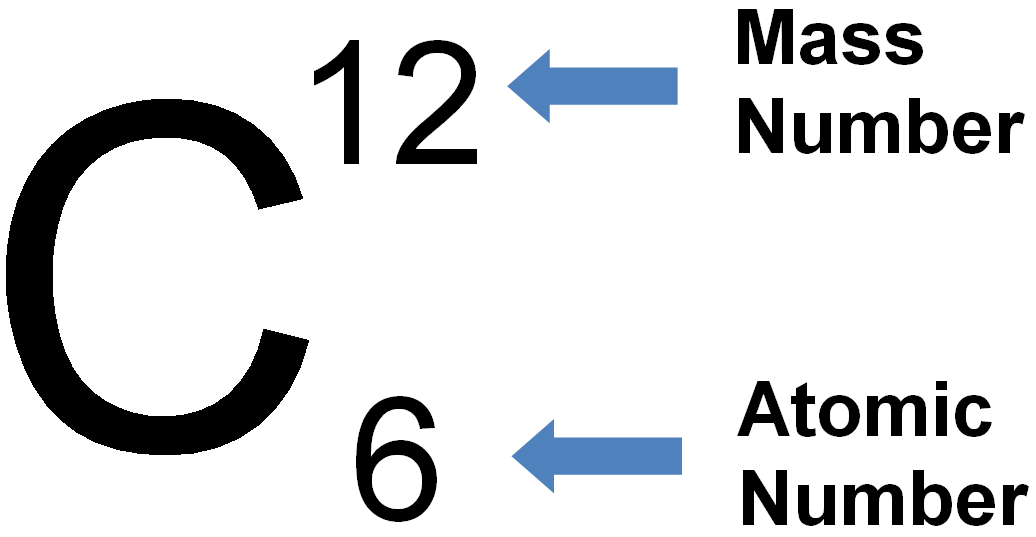
Now, because the atom has 53 electrons, it must also have 53 protons, and to find the number of neutrons we subtract this from the mass number. # n = A - # p = 127 - 53 = 74 neutrons. To summarize, you need to remember these relationships between the atomic mass, the number of protons, neutrons, and electrons:

No Atom Sign On White Background Stock Vector (Royalty Free) 182025116 Shutterstock
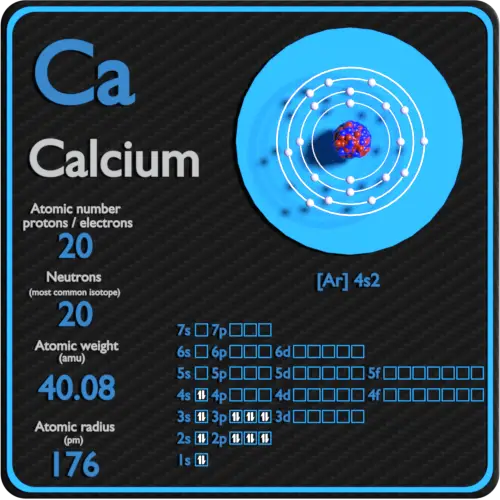
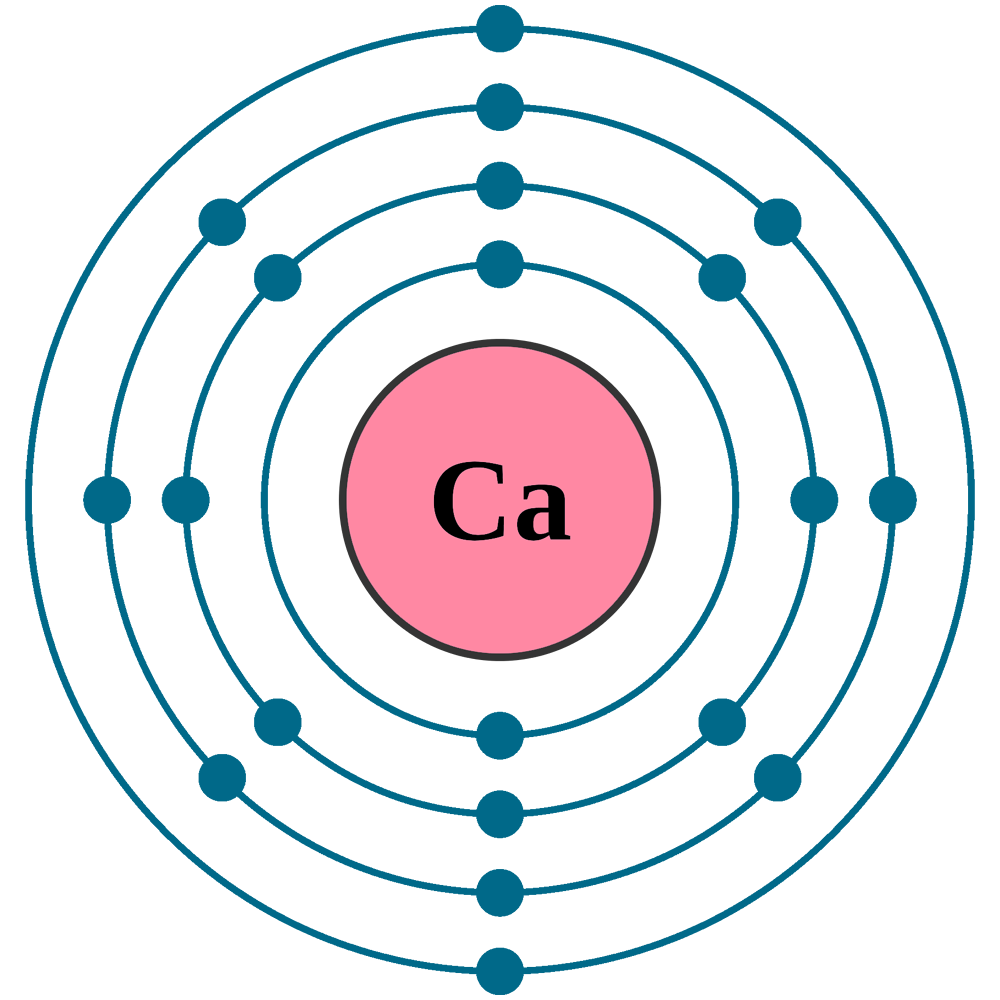
In order to write the Calcium electron configuration we first need to know the number of electrons for the Ca atom (there are 20 electrons). When we write the configuration we'll put all 20 electrons in orbitals around the nucleus of the Calcium atom. In writing the electron configuration for Calcium the first two electrons will go in the 1s.
Calcio Tabla periódica y propiedades atómicas
20, 20, 20 Assuming that we are referring to a stable atom of calcium, we need to find the atomic number of calcium. This can be memorized, or found on the periodic table. Calcium is the 20th element, with 20 protons (since the number of protons directly changes the element itself). Since a stable atom has a net charge of 0, we must have 20 electrons. The number of neutrons will be the same as.

Science and management of Ca and Mg
Daftar unsur menurut nomor atom. Berikut adalah daftar unsur kimia, diurutkan berdasarkan nomor atom, dan warna menunjukkan jenis unsur. Setiap unsur ditampilkan informasi mengenai nama unsur, lambang unsur, golongan dan periode, massa atom, massa jenis, titik lebur, titik didih dan penemunya.
Modelo De Bohr Del Calcio rudenko
A Because sulfur is to the left of fluorine in the periodic table, sulfur is named first. Because there is only one sulfur atom in the formula, no prefix is needed. B There are, however, six fluorine atoms, so we use the prefix for six: hexa - ( Table 2.12. 2 ). The compound is sulfur hexafluoride.
Example Cuantos Electrones Tiene El Calcio Png Cismos My XXX Hot Girl
In this video we'll look at the atomic structure and Bohr model for the Calcium atom (Ca). We'll use a Bohr diagram to visually represent where the electrons.

Calcium Mind Map
Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form CaCl 2, which is composed of Ca 2+ and Cl − ions in the ratio of one Ca 2+ ion to two Cl − ions. A compound that contains ions and is held together by ionic bonds is called an ionic compound. The periodic table can help us.

Cara mudah menentukan nomor atom dari bilangan kuantum Kimia SMA YouTube
Therefore, this new particle is no longer a calcium (Ca) atom which, as stated above, contains 20 electrons. This particle is now an ion, a charged particle, as a result of the imbalance between the number of positively-charged protons (+20) and negatively-charged electrons (-18) that it possesses. This particle now contains two more.

Nomor Atom, Nomor Massa, dan Konfigurasi Elektron YouTube
To find the number of atoms in Ca(NO3)2 , Calcium Nitrate, we'll add up the number of atoms for Ca, N, and O. The small number after the element symbol is c.
Sin signo atómico con frases en vector de stock (libre de regalías) 1882957660 Shutterstock
In this case, the calcium atom carries a positive charge. Ca - 2e - → Ca 2+. Here, the electron configuration of calcium ion (Ca 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6. This positive calcium ion (Ca 2+) has twenty protons, twenty neutrons, and eighteen electrons. Calcium ion.
No Atom Prohibition Sign Icon Illustration Stock Vector (Royalty Free) 391055932 Shutterstock
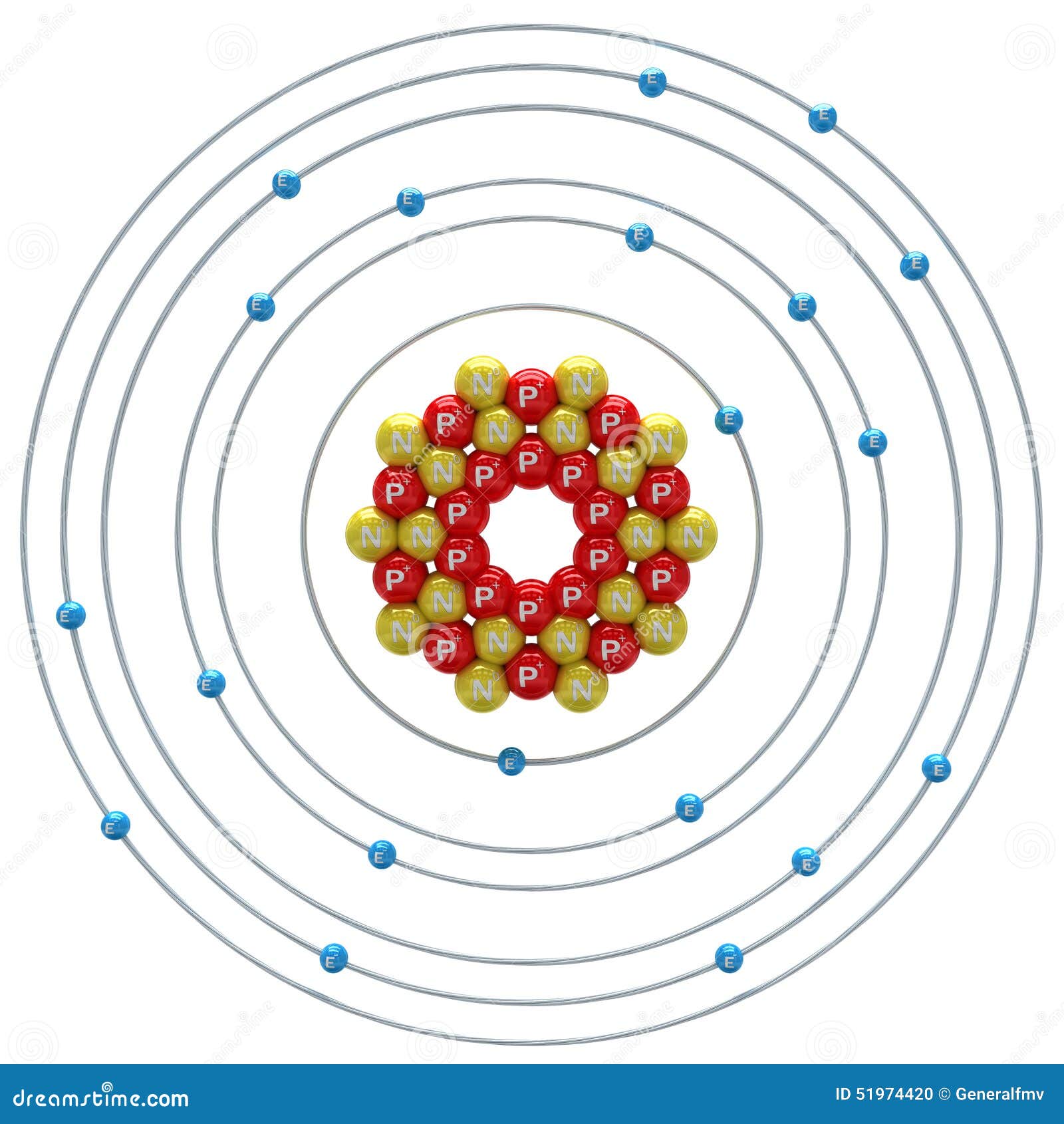
Atomic Number of Calcium. Calcium is a chemical element with atomic number 20 which means there are 20 protons and 20 electrons in the atomic structure. The chemical symbol for Calcium is Ca. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons.

Electron Configuration for Calcium (Ca, Ca2+ ion)
Calcium is a chemical element with atomic number 20 which means there are 20 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.

Nucleons, Atomic Number and Mass Number Definition [with Examples]
Let us determine the Lewis structures of SiH 4, CHO 2 −, NO +, and OF 2 as examples in following this procedure: Determine the total number of valence (outer shell) electrons in the molecule or ion. For a molecule, we add the number of valence electrons on each atom in the molecule: SiH 4 Si: 4 valence electrons/atom × 1 atom = 4 + H: 1.