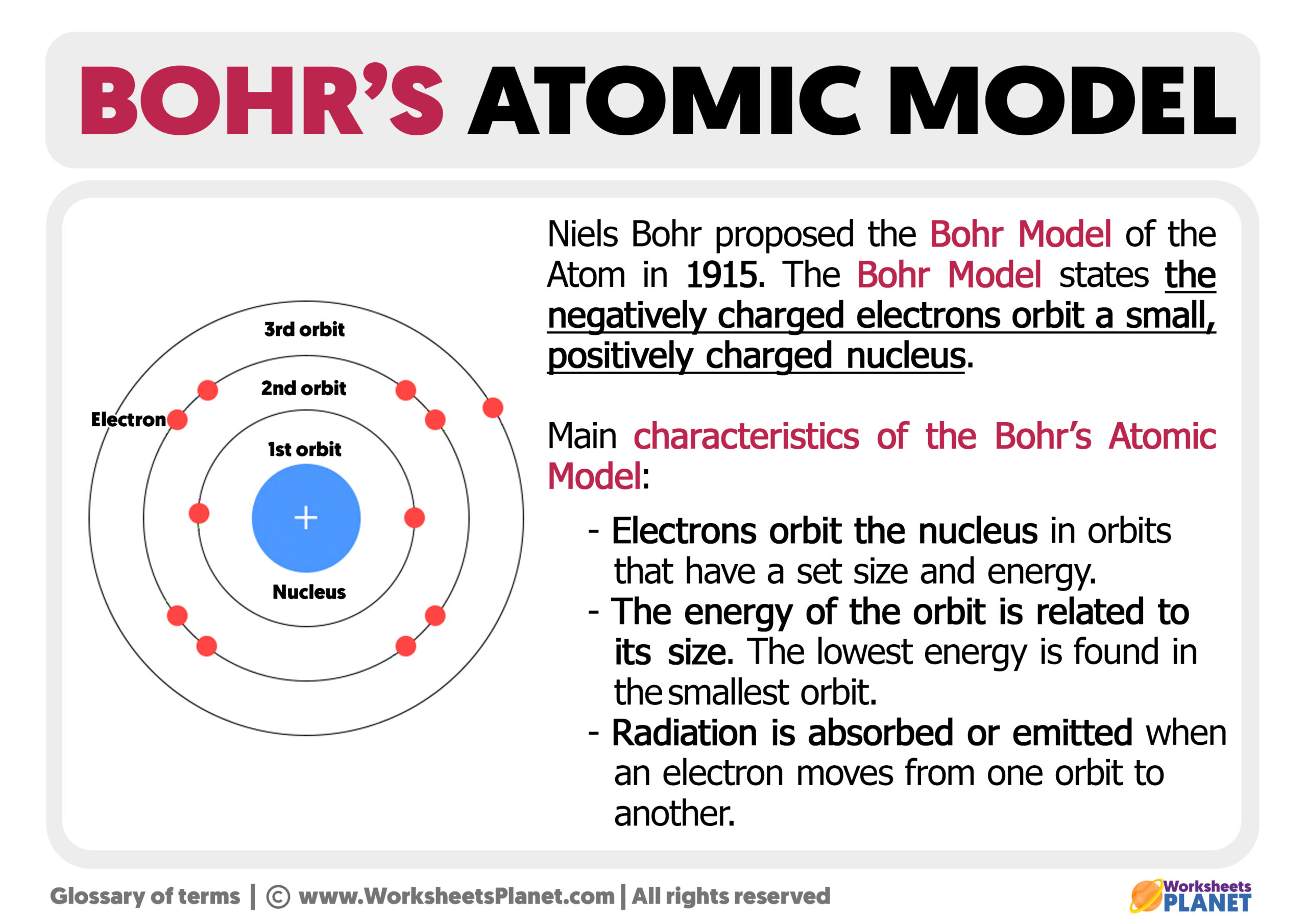
Bohr's Atomic Model
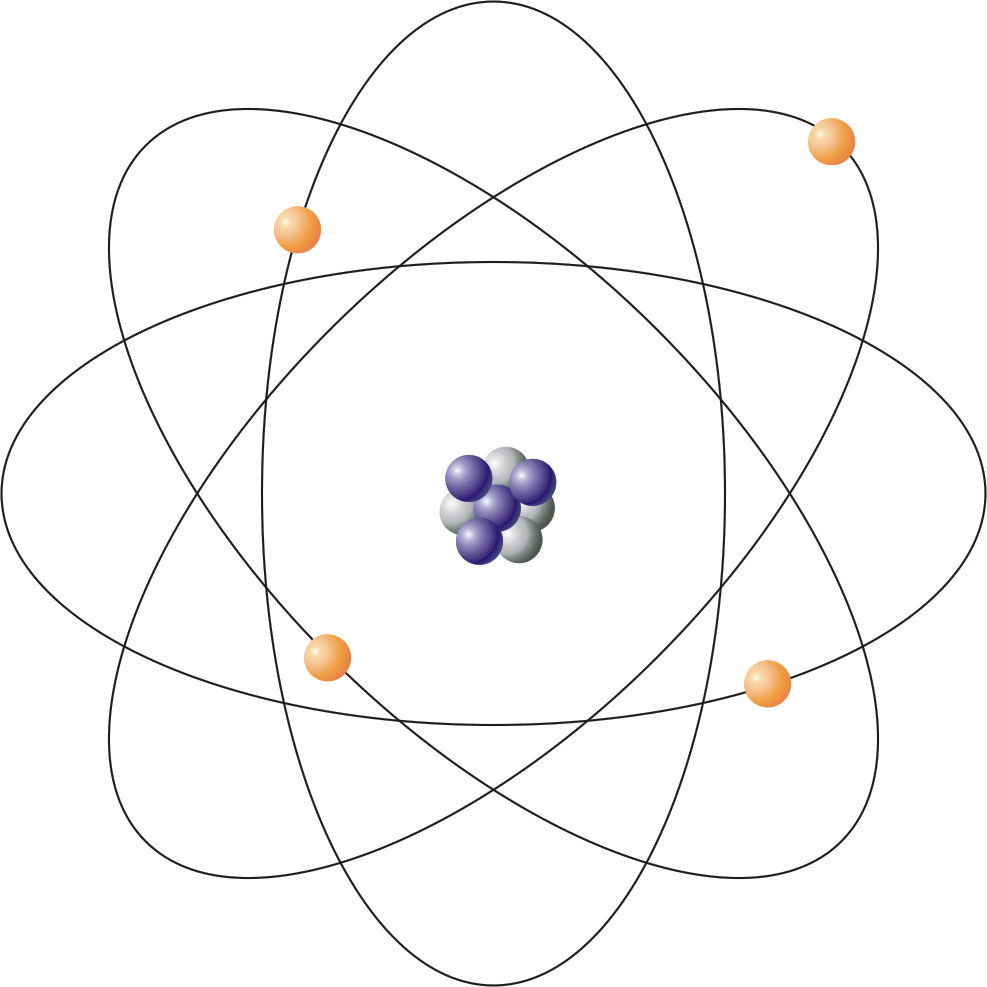
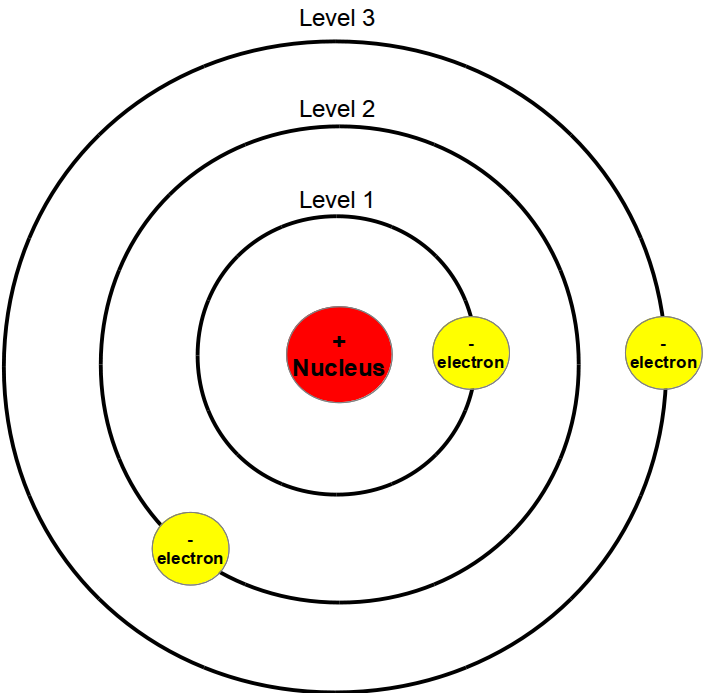
The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Only certain electron orbits are permitted.

Actualizar 88+ imagen cual fue el modelo atomico de niels bohr Thcshoanghoathambadinh.edu.vn
Niels Bohr (1885-1962) was a Danish physicist and winner of the 1922 Nobel Prize in Physics. Bohr began his work on the Manhattan Project after fleeing to Sweden from Denmark because of German occupation in 1943.. the most widely accepted model of the atom. In 1922, Bohr was awarded the Nobel Prize in Physics for his research and.
PPT Bohr’s Atomic Theory PowerPoint Presentation, free download ID6914705
Niels Bohr was born on October 7, 1885, in Copenhagen, Denmark, to mother Ellen Adler, who was part of a successful Jewish banking clan, and father Christian Bohr, a celebrated physiology academic.
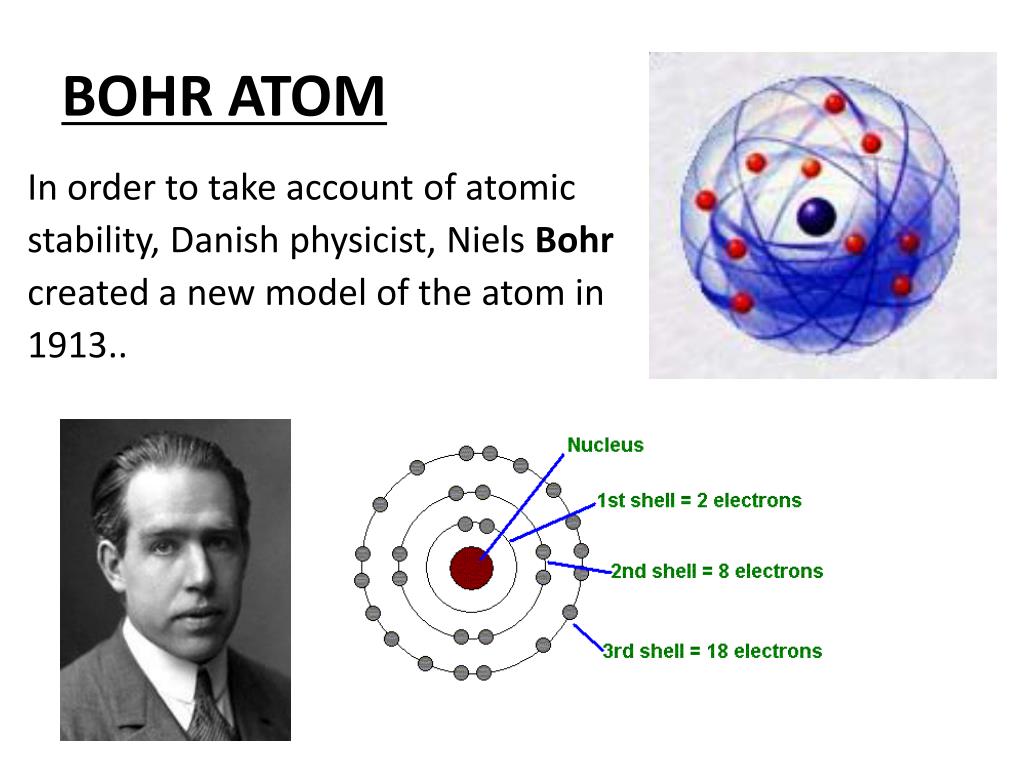
PPT The History of the Atom PowerPoint Presentation, free download ID7090994
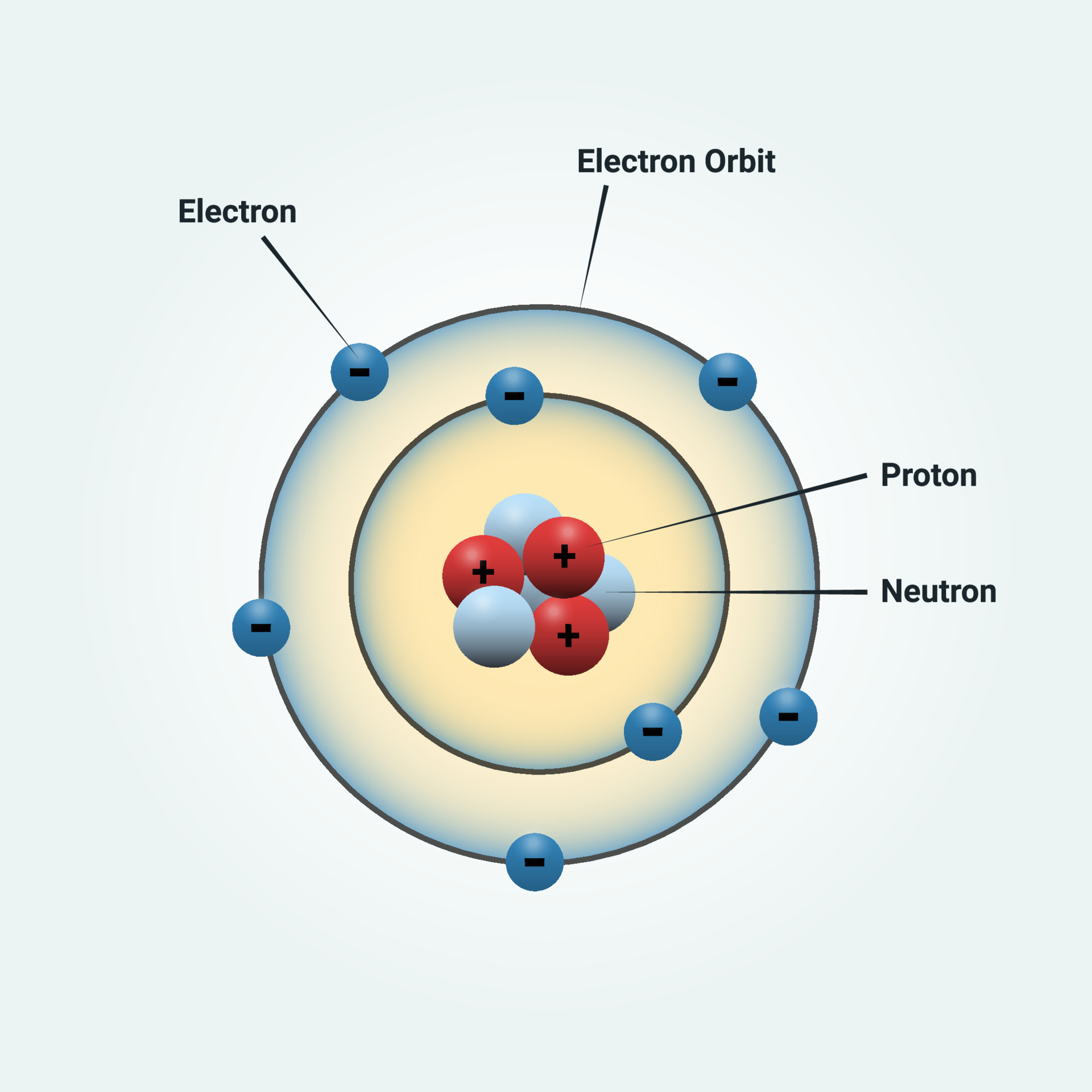
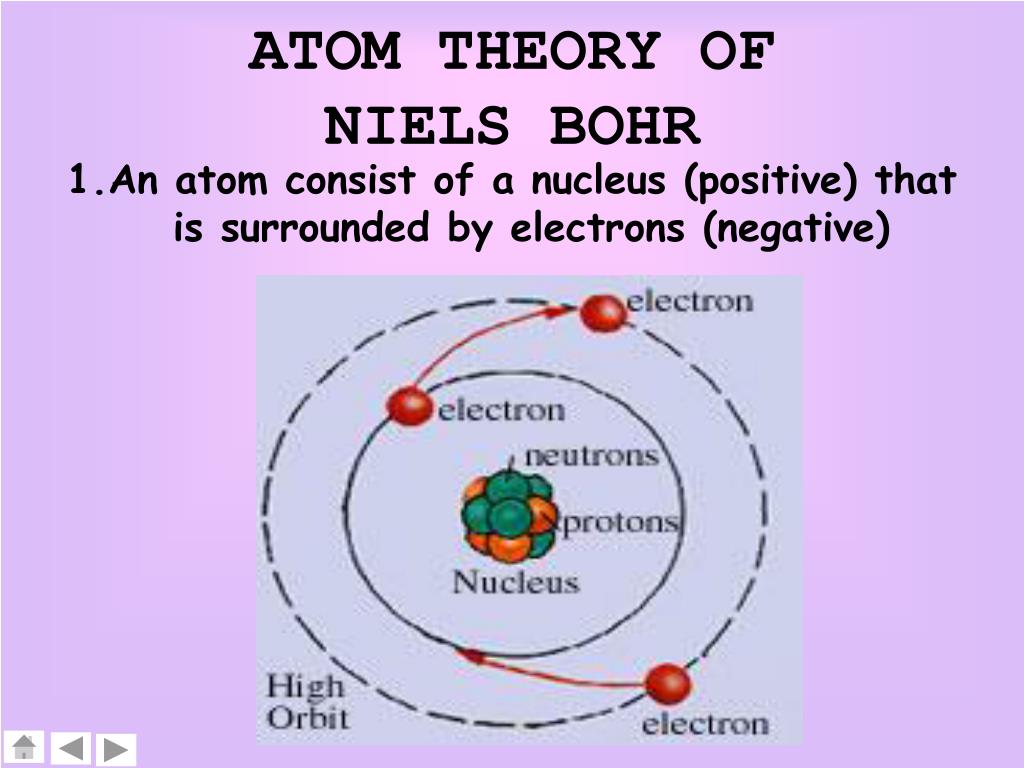
What is Bohr's Model of an Atom? The Bohr model of the atom was proposed by Neil Bohr in 1915. It came into existence with the modification of Rutherford's model of an atom. Rutherford's model introduced the nuclear model of an atom, in which he explained that a nucleus (positively charged) is surrounded by negatively charged electrons.
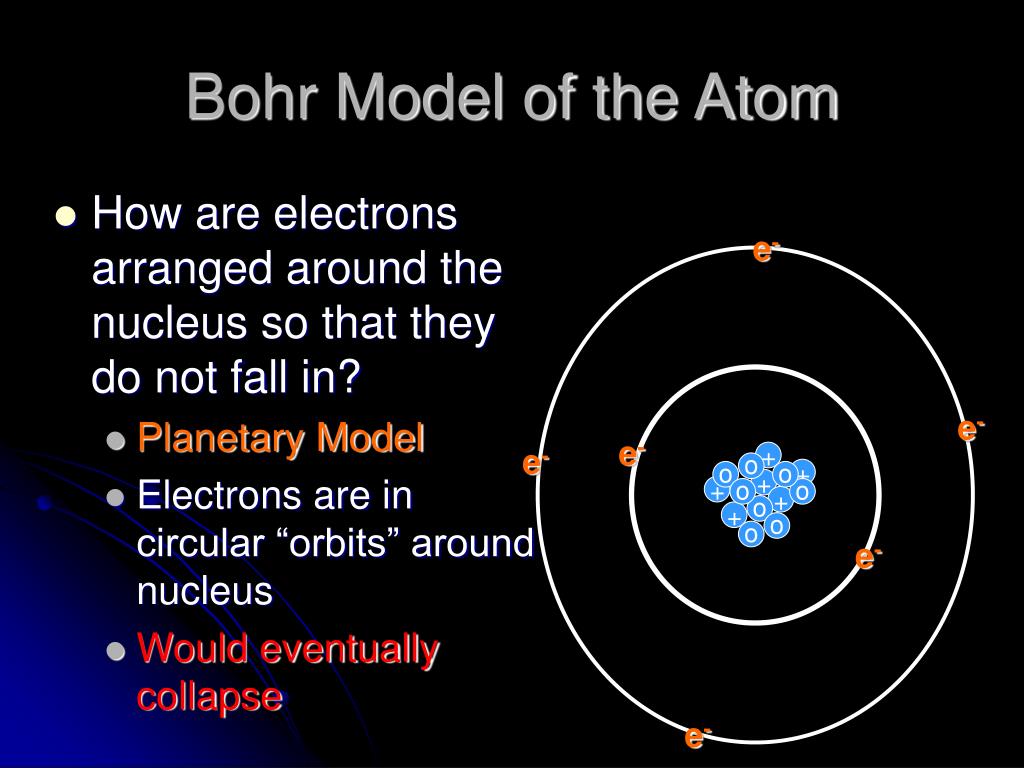
PPT Bohr Model of the Atom PowerPoint Presentation, free download ID6575494
Niels Bohr (born October 7, 1885, Copenhagen, Denmark—died November 18, 1962, Copenhagen) Danish physicist who is generally regarded as one of the foremost physicists of the 20th century.He was the first to apply the quantum concept, which restricts the energy of a system to certain discrete values, to the problem of atomic and molecular structure. . For that work he received the Nobel Prize.
Bohr atomic model of a nitrogen atom. vector illustration for science 4511236 Vector Art at Vecteezy
Niels Bohr, (born Oct. 7, 1885, Copenhagen, Den.—died Nov. 18, 1962, Copenhagen), Danish physicist.He studied the structure of the atom with J.J. Thomson and Ernest Rutherford at the universities of Cambridge and Manchester. He was among the first to see the importance of an element's atomic number and postulated that any atom could exist only in a discrete set of states characterized by.

Atómico Modelo Bohr Imagen gratis en Pixabay Pixabay
Bohr's Atomic Model. Following the discoveries of hydrogen emission spectra and the photoelectric effect, the Danish physicist Niels Bohr (1885-1962) proposed a new model of the atom in 1915. Bohr proposed that electrons do not radiate energy as they orbit the nucleus, but exist in states of constant energy that he called stationary states.
Chemistry Glossary Search results for 'Bohrov atom'
1 λ = R( 1 n2f − 1 n2i) (30.3.21) (30.3.21) 1 λ = R ( 1 n f 2 − 1 n i 2) We see that Bohr's theory of the hydrogen atom answers the question as to why this previously known formula describes the hydrogen spectrum. It is because the energy levels are proportional to 1/n2 1 / n 2, where n n is a non-negative integer.
PPT ATOMIC STRUCTURE PowerPoint Presentation, free download ID4498481
John L. Heilbron describes the route that led Niels Bohr to quantize electron orbits a century ago. In the autumn of 1911, the Danish physicist Niels Bohr set sail for a postdoctoral year in.
Unreal Truths Matter Waves and the Bohr Model of the Atom
The atom would radiate a photon when an excited electron would jump down from a higher orbit to a lower orbit. The difference between the energies of those orbits would be equal to the energy of the photon. Niels Bohr was a Danish physicist and is considered one of the founding fathers of quantum mechanics, precisely old quantum mechanics. For.

Bohr model Description & Development Britannica
In 1913, Niels Bohr proposed a theory for the hydrogen atom, based on quantum theory that some physical quantities only take discrete values. Electrons move around a nucleus, but only in prescribed orbits, and If electrons jump to a lower-energy orbit, the difference is sent out as radiation.
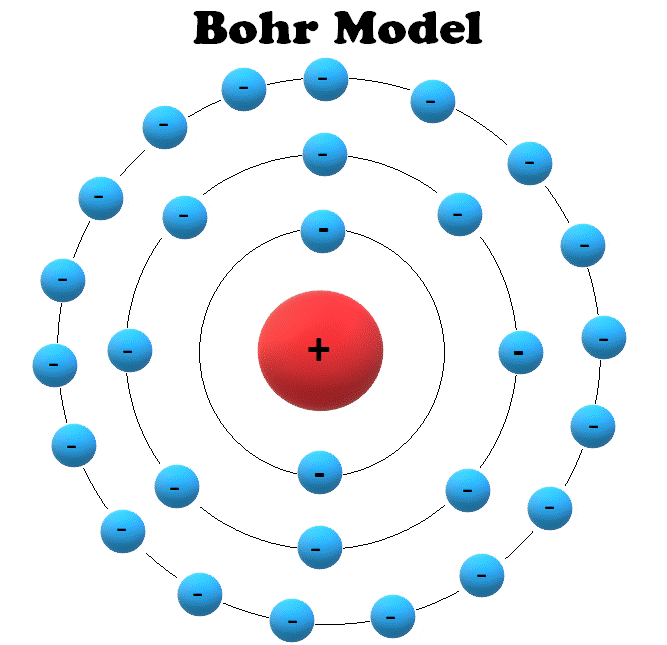
Atoms and Electrons Electronics Reference
Niels Henrik David Bohr (Danish: [ˈne̝ls ˈpoɐ̯ˀ]; 7 October 1885 - 18 November 1962) was a Danish physicist who made foundational contributions to understanding atomic structure and quantum theory, for which he received the Nobel Prize in Physics in 1922. Bohr was also a philosopher and a promoter of scientific research.. Bohr developed the Bohr model of the atom, in which he proposed.
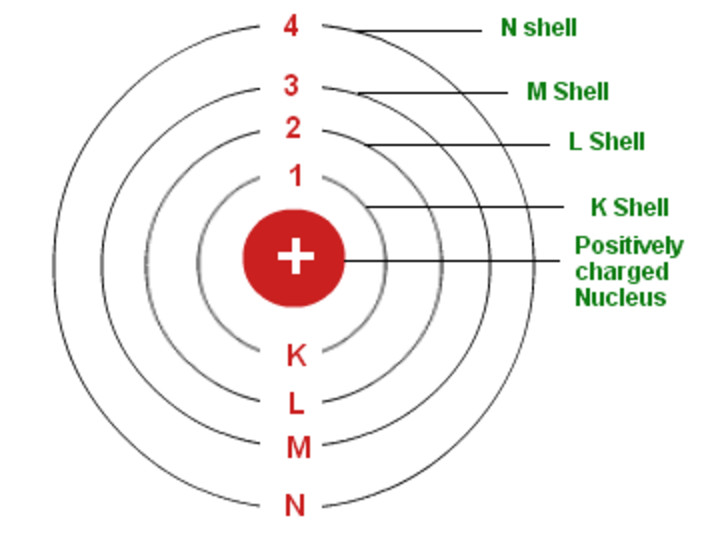
Bohr's Model of an Atom Chemistry, Class 11, Structure of Atom
Niels Bohr Biographical . N iels Henrik David Bohr was born in Copenhagen on October 7, 1885, as the son of Christian Bohr, Professor of Physiology at Copenhagen University, and his wife Ellen, née Adler. Niels, together with his younger brother Harald (the future Professor in Mathematics), grew up in an atmosphere most favourable to the development of his genius - his father was an eminent.
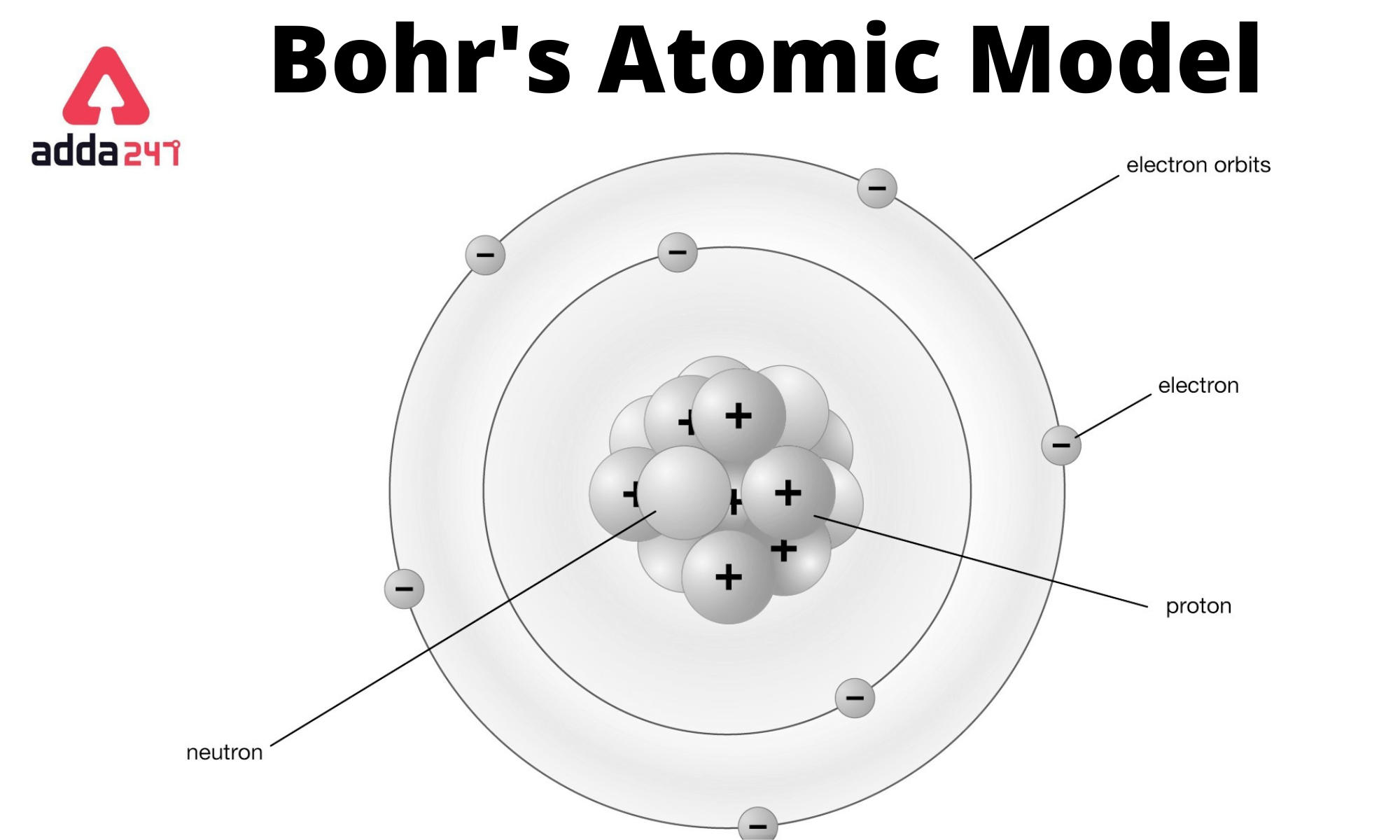
Bohr Atomic Model Formula, Postulates and Limitations, Diagram
Bohr's model calculated the following energies for an electron in the shell, n. . : E ( n) = − 1 n 2 ⋅ 13.6 eV. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the photon energy is. h ν = Δ E = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 eV.
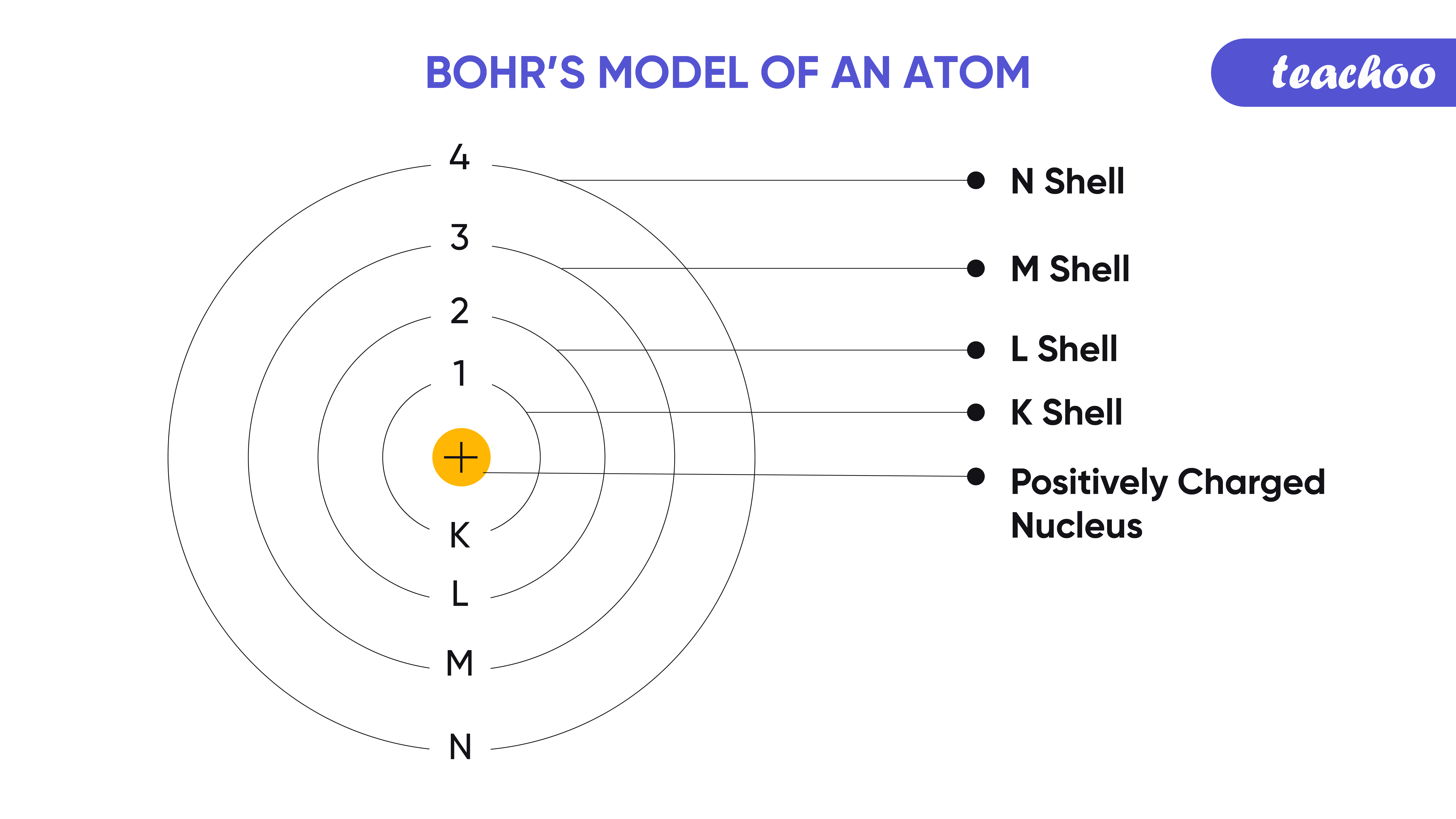
Bohrs Model Of An Atom With Postulates And Limitations Of Bohrs Model My XXX Hot Girl
In 1913, Niels Bohr proposed Bohr's theory using the spectral lines of the hydrogen atom and Planck's quantum theory. In light of this information, Bohr's postulates can be summarized as follows:

Describe Bohrs Model of the Atom
Aage N. Bohr, (born June 19, 1922, Copenhagen, Den.—died Sept. 8, 2009, Copenhagen), Danish physicist who shared the 1975 Nobel Prize for Physics with Ben R. Mottelson and James Rainwater for their work in determining the asymmetrical shapes of certain atomic nuclei. Bohr was educated at the University of Copenhagen, where he received a.