
Persamaan Ionisasi Senyawa Elektrolit Dalam Air Berikut Yang Benar Adalah Hot Sex Picture
Add a comment. -1. The products will be HNOX3 +NHX4OH H N O X 3 + N H X 4 O H. This is because a salt hydrolysis reaction always produces an acid and a base. The HX+ H X + in HX2O H X 2 O will bond with NOX3X− N O X 3 X − to form HNOX3 H N O X 3, a strong acid. The remaining OHX− O H X − in HX2O H X 2 O will bond with NHX4X+ N H X 4 X.

AgCl + HNO3 = ??? (No reaction) YouTube
The following equation is used for calculating acid and base molarity where the concentration is given in wt %: [ (% × d) / MW] × 10 = Molarity. Where: % = Weight %; d = Density (or specific gravity); MW = Molecular Weight (or Formula Weight). The above equation can then be used to calculate the Molarity of the 70 wt % Nitric Acid:

NH4OH là gì? Đặc điểm tính chất ứng dụng Amoni hydroxit
Balance HNO3 + NH3OH = H2O + NH4NO3 Using the Algebraic Method To balance the equation HNO3 + NH3OH = H2O + NH4NO3 using the algebraic method step-by-step, you must have experience solving systems of linear equations.

Амоніак та його властивості презентація з хімії
When ammonium hydroxide (NH4OH) and nitric acid (HNO3) are combined, the ammonium ion (NH4+) from the ammonium hydroxide swaps places with the hydrogen ion (H+) from the nitric acid. This results in the formation of ammonium nitrate, which is a white crystalline solid that is commonly used in fertilizers, explosives, and in various industrial.

NH4OH + HNO3 → NH4NO3 + H2O Реакция гидрата аммиака и азотной кислоты YouTube
Since there is an equal number of each element in the reactants and products of HNO3 + NH4OH = N2O + 3H2O, the equation is balanced. Balance HNO3 + NH4OH = N2O + H2O Using Inspection The law of conservation of mass states that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a chemical.

NH4OH Lewis Dot Structure (Ammonium Hydroxide) YouTube
In this video we'll balance the equation NH4OH + HNO3 = NH4NO3 + H2O and provide the correct coefficients for each compound.To balance NH4OH + HNO3 = NH4NO3.

Ejercicio 5 de Ácidos y Bases. Una base débil, el NH4OH YouTube
This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer. Question: Balance the following acid-base neutralization reaction. [Select] [Select] NH4OH (aq) + [ Select] NH4NO3 (aq) + [Select] 4 Note: Use the blank option for a value of 1. V HNO3 (aq) → H₂O (l)
Solutions. Acidbase equilibrium in biological systems презентация онлайн
Balance NH4OH + HNO3 = NHNO3 + H2O Using Inspection The law of conservation of mass states that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a chemical reaction as at the beginning.

SOLVED The heat of neutralisation is highest for the reaction between A. NH4OH + CH3COOH B
There are three main steps for writing the net ionic equation for AgNO3 + NH4OH = AgOH + NH4NO3 (Silver nitrate + Ammonium hydroxide). First, we balance the.

Ammonium hydroxide, ammonia solution, NH4OH molecule. Skeletal chemical formula. Vector
NH4OH*HNO3 molar mass. Molar mass of NH4OH*HNO3 is 98.0586 g/mol. Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between NH4OH*HNO3 weight and moles. Compound.
NH4OH Là Gì ? Tính Chất Và Ứng Dụng Quan Trọng Hoá Chất Trần Tiến
Description. Ammonium hydroxide appears as a colorless aqueous solution. Concentration of ammonia ranges up to approximately 30%. Ammonia vapors (which arise from the solution) irritate the eyes. CAMEO Chemicals. Ammonium hydroxide is a solution of ammonia in water. It has a role as a food acidity regulator. ChEBI.

How to balance NH4OH+HNO3=NH4NO3=+H2OReaction balance NH4OH+HNO3=NH4NO3+H2ONH4OH+HNO3=NH4NO3
There are three main steps for writing the net ionic equation for HNO3 + NH4OH = NH4NO3 + H2O (Nitric acid + Ammonium hydroxide). First, we balance the molec.

NH4OH AMMONIUM HYDROXIDE 0935161826
Check the balance. Now, both sides have 4 H atoms and 2 O atoms. The equation is balanced. Balancing with algebraic method. This method uses algebraic equations to find the correct coefficients.

Al Oh 3 Hno3 Estudiar
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. Since there is an equal number of each element in the reactants and products of NH4OH + HNO3 = NH4NO3 + H2O, the equation is balanced.

How to Balance NH4OH + HNO3 = NH4NO3 + H2O (Ammonium hydroxide + Nitric acid) YouTube
Free Chemical Reactions calculator - Calculate chemical reactions step-by-step
Nh4oh hires stock photography and images Alamy
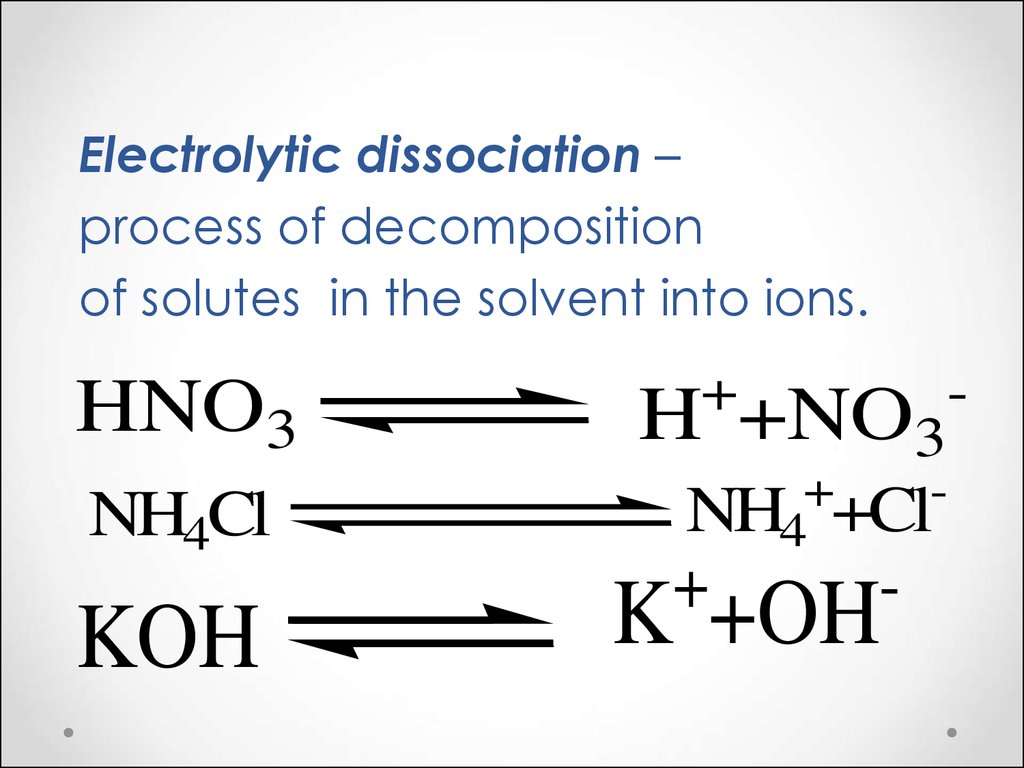
You can either write $\ce{NH4OH}$ more conventionally (or in a more modern way) as ammonia in aqueous solution, $\ce{NH3(aq)}$, which is a weak base. Or you can recognize $\ce{OH-}$ in ammonium hydroxide as a strong base, which would react with the ammonium to make ammonia. In either way of looking at things, the conjugate base is present as well.