
Fosfato Monosódico De La Molécula NaH2PO4 Ilustración del Vector Ilustración de molecular
H3PO4 + Na2HPO4 = NaH2PO4 is a Synthesis reaction where one mole of aqueous Phosphoric Acid [H 3 PO 4] and one mole of aqueous Dibasic Sodium Phosphate [Na 2 HPO 4] combine to form two moles of aqueous Monosodium Phosphate [NaH 2 PO 4] Show Chemical Structure Image. Reaction Type. Synthesis.

Sodium Dihydrogen Phosphate dihydrate (NaH2PO4.2H2O) Hóa Chất Thí Nghiệm
Word Equation. Monosodium Phosphate + Dibasic Sodium Phosphate = Sodium Tripolyphosphate + Water. NaH2PO4 + Na2HPO4 = Na5P3O10 + H2O is a Double Displacement (Metathesis) reaction where one mole of aqueous Monosodium Phosphate [NaH 2 PO 4] and two moles of aqueous Dibasic Sodium Phosphate [Na 2 HPO 4] react to form one mole of aqueous Sodium Tripolyphosphate [Na 5 P 3 O 10] and two moles of.

RPI SodiumPhosphate Monobasic Monohydrate S23120, 10049215, 138, NaH2PO4 • H2O, Buffers
Sodium dihydrogen phosphate reacts with bases like sodium hydroxide resulting in the formation of sodium hydrogen phosphate and water. NaH2PO4 + NaOH → Na2HPO4 + H2O. Sodium dihydrogen phosphate reacts with acid like hydrochloric acid results in the formation of phosphoric acid and sodium chloride. NaH2PO4 + HCl → H3PO4 + NaCl.

NaH2PO4 Sodium Acid Phosphate Anhydrous Widely Use In Food Industry
NaH2PO4, also known as sodium dihydrogen phosphate, is an important compound with various characteristics. One of these characteristics is its pH and pKa value. The pH of a solution refers to its acidity or alkalinity, while pKa is a measure of the acidity of an acid. In the case of NaH2PO4, it acts as a weak acid.

NaH2PO4 Monosodium PhosphateNatri đihiđrophotphatE
We have this exercise without solutions. From a 0.2 M $\ce{NaH2PO4}$ solution and a 0.2 M $\ce{Na2HPO4}$ solution, a buffer solution with pH = 6.8 is to be prepared. The total concentration of $\ce{PO_4^3}$ - should be 0.1 mol l-¹. Calculate the volumes of the two solutions needed to prepare one litre of buffer solution.

Monosodium Phosphate [NaH2PO4] 98+ AR Grade Powder 1 Lb in Two Bottles USA
NaH2PO4 yang bersifat asam akan mendonasikan H+ bebas ketika [H+] turun, sedangkan NaH2PO4 yang bersifat basa akan menerima H+ bebas ketika [H+] naik. Secara reaksi kimia, hal ini dinyatakan sebagai berikut.2 NaH2PO4 + H+ NaH2PO4 + Na+ Perlu diketahui bahwa konsentrasi buffer fosfat di CES sangat rendah karena fosfat sangat banyak di dalam sel.

Dihyrogen Phosphate de sodium monohydraté Nah2PO4· H2O Chine Mono Phosphate de sodium
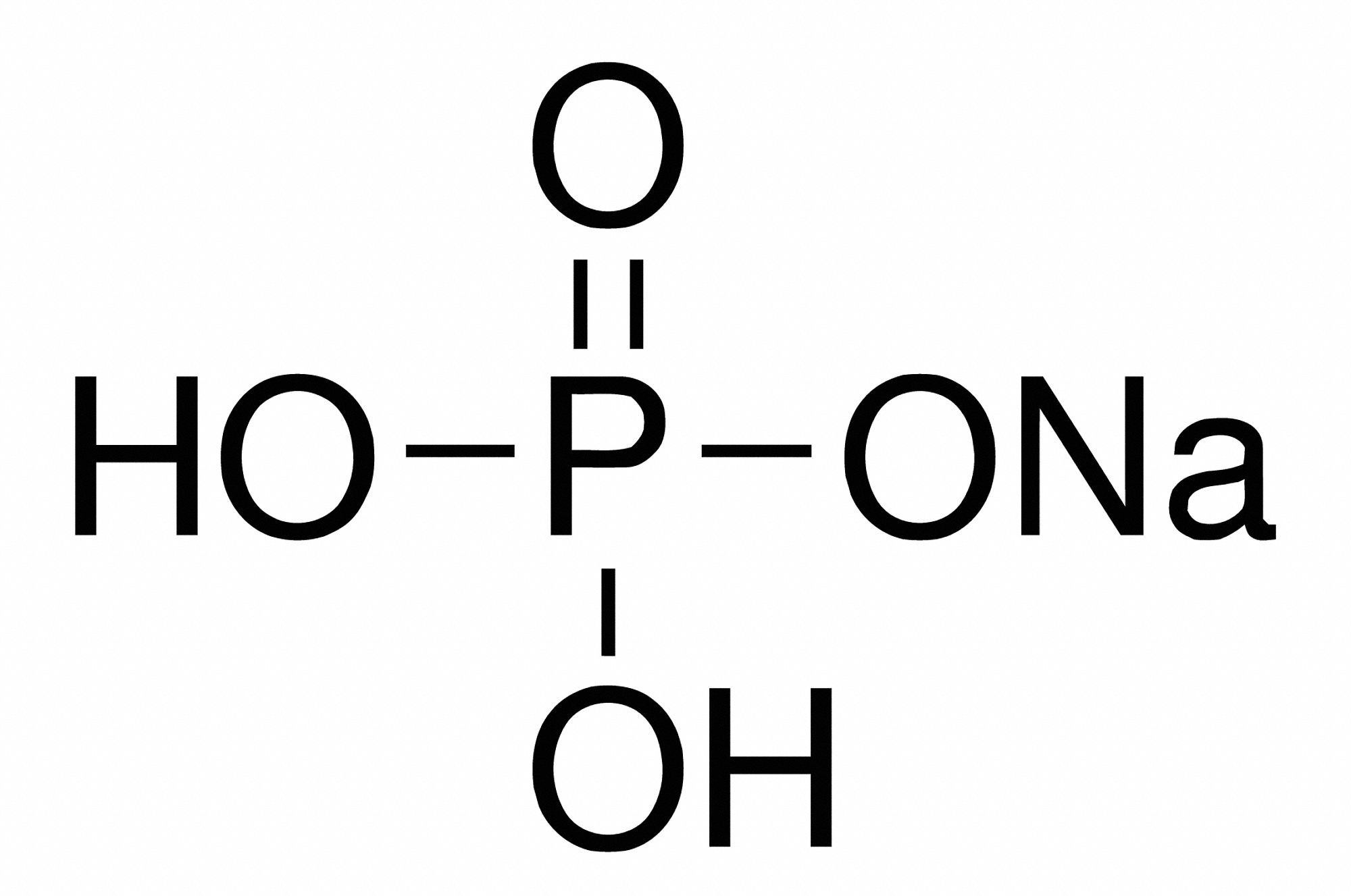
Monosodium phosphate (MSP), also known as monobasic sodium phosphate and sodium dihydrogen phosphate, is an inorganic compound with the chemical formula Na H 2 P O 4.It is a sodium salt of phosphoric acid.It consists of sodium cations (Na +) and dihydrogen phosphate anions (H 2 PO − 4).One of many sodium phosphates, it is a common industrial chemical.The salt exists in an anhydrous form, as.

NaH2PO4 Merck / Sodium Phosphate Monobasic Merck 10gr Lazada Indonesia
In extracellular fluid, the bulk of phosphate exists in inorganic form as the 2 constituents, NaH2PO4 and Na2HPO4; the ratio of disodium to monosodium phosphate is 4:1 at pH 7.4. This ratio varies with pH; however, due to its relatively low concn, phosphate contributes little to the buffering capacity of extracellular fluid.
SIGMAALDRICH Sodium Phosphate Monobasic Greater Than 99 Concentration, 7558807, 119.98
no: f/qcl/008 rev.01 pt.smart-lab indonesia lembar data keselamatan bahan - sodium phosphate dibasic heptahydrate page 2 lembar data keselamatan bahan

NaH2PO4 Monosodium Phosphate CAS Chemical Substance in White Plastic Laboratory Packaging Stock
Disodium phosphate (DSP), or disodium hydrogen phosphate, or sodium phosphate dibasic, is an inorganic compound with the chemical formula Na 2 H P O 4.It is one of several sodium phosphates.The salt is known in anhydrous form as well as hydrates Na 2 HPO 4 ·nH 2 O, where n is 2, 7, 8, and 12. All are water-soluble white powders. The anhydrous salt is hygroscopic.. The pH of disodium hydrogen.

How to find the Oxidation Number for P in NaH2PO4 (Sodium hydrogen phosphate) YouTube
Since there is an equal number of each element in the reactants and products of NaH2PO4*2H2O = NaH2PO4 + 2H2O, the equation is balanced. Balance NaH2PO4*2H2O = NaH2PO4 + H2O Using Inspection The law of conservation of mass states that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a chemical.

Monosodium Phosphate Anhydrous, Chemical Formula NaH2PO4, Crystal at Rs 104/kg in Delhi
Ph Eur. Sodium dihydrogen phosphate monohydrate MSDS (material safety data sheet) or SDS, CoA and CoQ, dossiers, brochures and other available documents. CAS #: 10049-21-5 EC Number: 231-449-2 Molar Mass: 137.99 g/mol Chemical Formula: NaH₂PO₄ * H₂O Hill Formula: H₂NaO₄P * H₂O Grade: ACS,Reag. Ph Eur.

Qualigens Sodium Dihydrogen Orthophosphate, Nah2po4 2h2o, Dihydrate at best price in New Delhi
In this study, solubility and physic-chemical properties of sodium dihydrogen phosphate in sodium chloride, phosphoric acid and their mixture solutions at T = (298.15 and 313.15) K have been investigated by using isothermal dissolution method.In the three systems, the solubility of NaH 2 PO 4 always increases with the temperature increasing and decreases with molar concentration of phosphoric.
Sodium phosphate NaH2PO42H2O 1kg Shopee Việt Nam
1 Answer. Monosodium dihydrogen phosphate, HX2POX4X− H X 2 P O X 4 X −, is an amphoteric species and it will act as both an acid and a base. These are the related chemical equations: HX2POX4X− +HX2O HX2POX4X− +HX2O ↽−−⇀HPOX4X2− +HX3OX+ ↽−−⇀HX3POX4 +OHX− pKa pKb = 7.21 = 11.88 H X 2 P O X 4 X − + H X 2 O ↽ − −.

How to assign OXIDATION NUMBER of Phosphorus in NaH2PO4 ? YouTube
Chemistry questions and answers. (W) Preparation of phosphate buffers NaH2PO4 + NaOH (acid) (base) Na2HPO4+H2O (salt) (water) For phosphate buffers, there is a simple procedure: one simply combines solutions of conjugate acid and conjugate base to reach the desired pH. Take 70 ml cach of these two 0.1 M phosphate solutions: acid (NaH2PO4 FW 156.
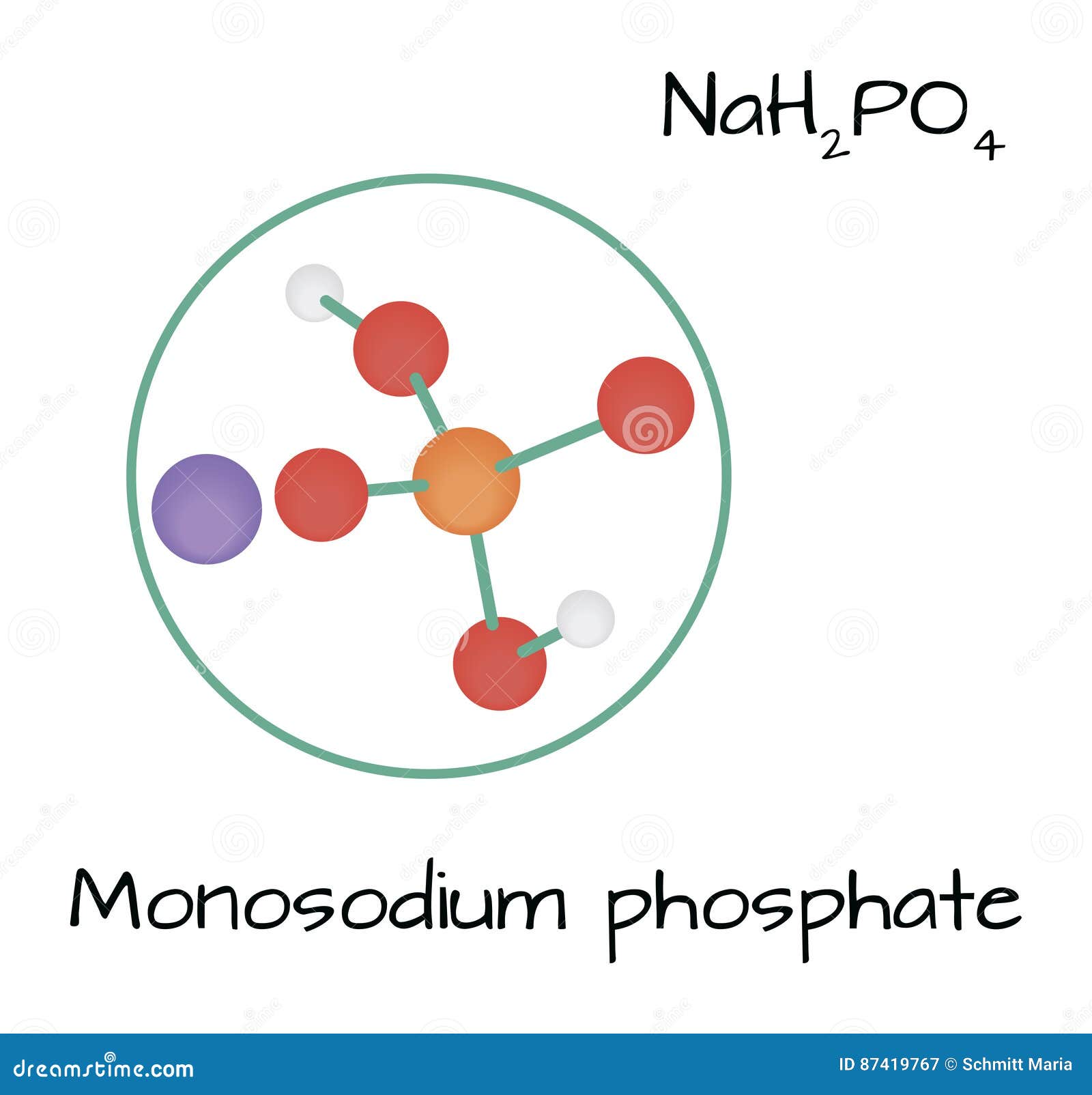
Molecule NaH2PO4 Monosodium Phosphate Stock Vector Illustration of vector, concept 87419767
15.9994. 3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in (NaH2PO4)2H2O: Molar Mass (g/mol) Na (Sodium) 2 × 22.98976928 = 45.97953856. H (Hydrogen) 6 × 1.00794 = 6.04764.