
Cara Super Cepat Menentukan Kepolaran Molekul, Bonus Cara Cepat Memprediksi Orbital Hibrida dan
Unlike polar bonds, non-polar bonds share electrons equally. A bond between two atoms or more atoms is non-polar if the atoms have the same electronegativity or a difference in electronegativities that is less than 0.4. An example of a non-polar bond is the bond in chlorine. Chlorine contains two chlorine atoms.
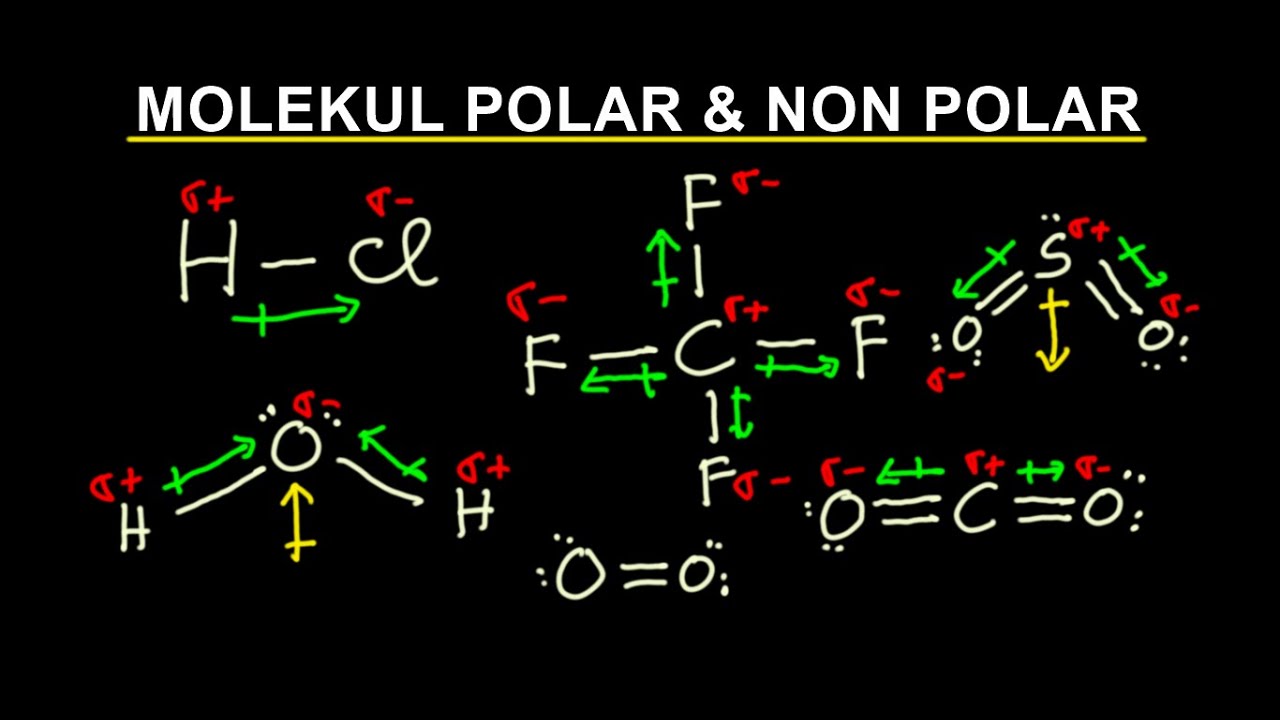
MOLEKUL POLAR & NON POLAR YouTube
Polar Molecules. A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. A diatomic molecule that consists of a polar covalent bond, such as \(\ce{HF}\), is a polar molecule. The two electrically charged regions on either end of the molecule are called poles, similar to a magnet having a north and a south pole.
VSEPR Theory and Polarity Kyle's Digital Portfolio
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.

Bentuk molekul berikut yang bersifat polar adalah... (bis...
A polar molecule is a chemical species in which the distribution of electrons between the covalently bonded atoms is not even. Polarity is a description of how different the electrical poles of a molecule are.
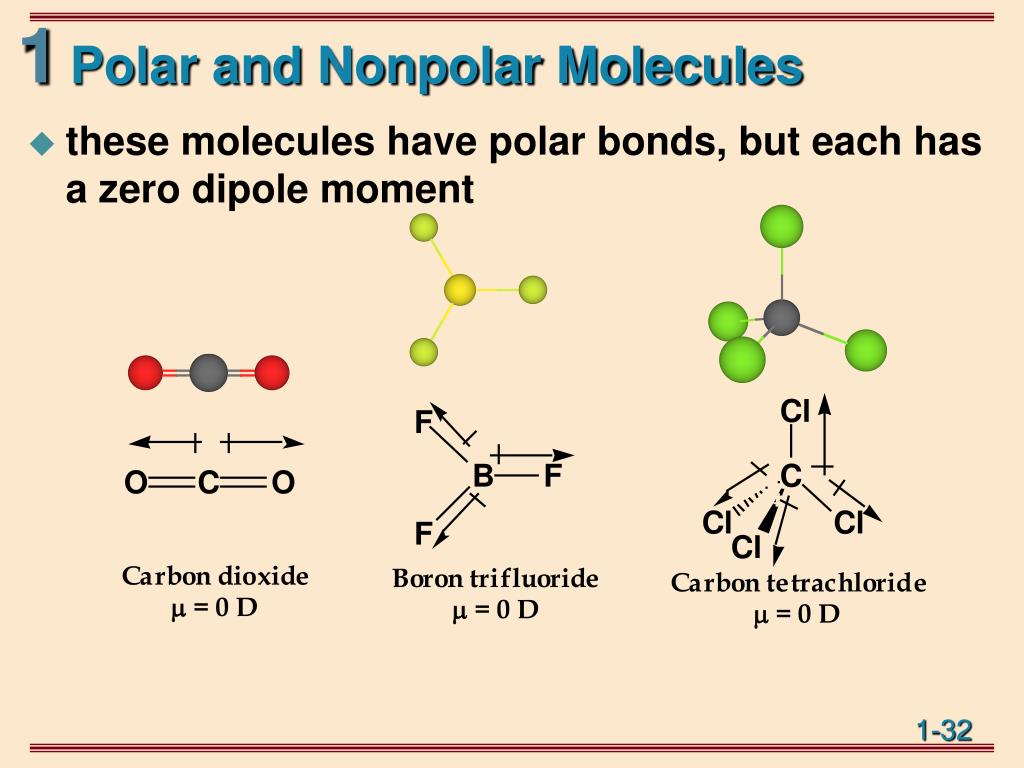
Ppt Polar Bonds And Molecules Powerpoint Presentation, Free Download FD6
Non-polar molecules: Hydrocarbons (gasoline, toluene), homo-nuclear diatomic molecules (O 2, N 2, Cl 2, H 2, etc.), noble gases, benzene, methane, ethylene, carbon tetrachloride How to Determine if a Molecule is Polar Or Nonpolar. Start by drawing its Lewis structure. This rule applies to all molecules except hydrocarbons and molecules with two atoms of the same element.

Moleküllerin Polarlık ya da Apolarlığı konu anlatımı ders notu 9.sınıf kimya
Ikatan Kimia (8) | Cara Menentukan Molekul Polar dan Non Polar | Kimia Kelas 10 Kimatika 103K subscribers Subscribe Subscribed 2.3K 98K views 3 years ago Kimia kelas 10 | Ikatan Kimia.
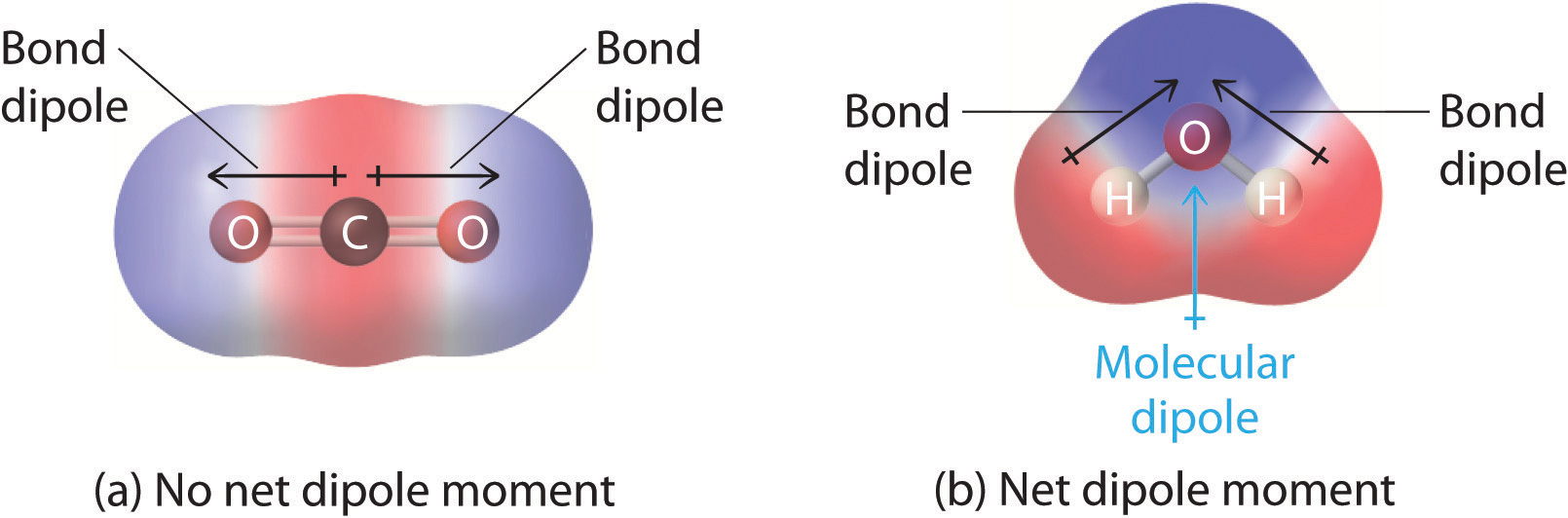
2.2 Polar Covalent Bonds Dipole Moments Chemistry LibreTexts
A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. A diatomic molecule that consists of a polar covalent bond, such as HF, is a polar molecule.
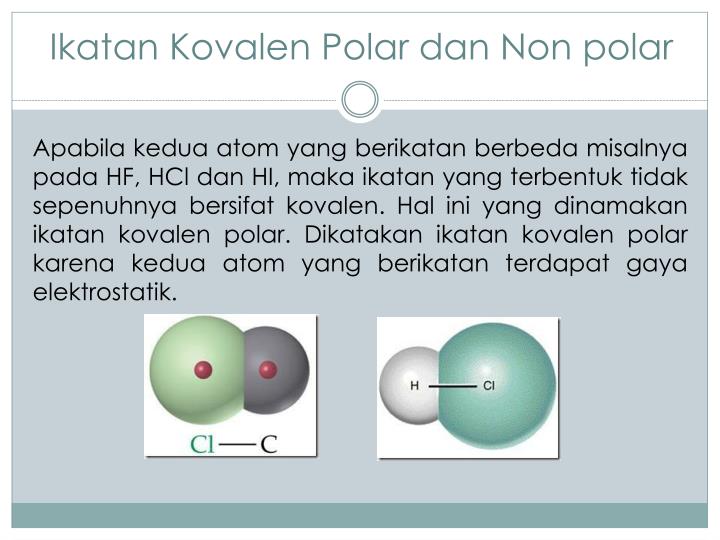
Ikatan Kovalen Polar Dan Nonpolar Beserta Contoh Ikatannya Rumus Kimia Sexiz Pix
The polarity of molecules is related to the polarity of bonds within the molecule, but just having polar bonds is not enough to create a polar molecule. Consider, for example, CCl 4 and CHCl 3. Carbon tetrachloride has 4 fairly polar bonds but they form a regular tetrahedron and the polarity of the individual bonds cancel each other out to.
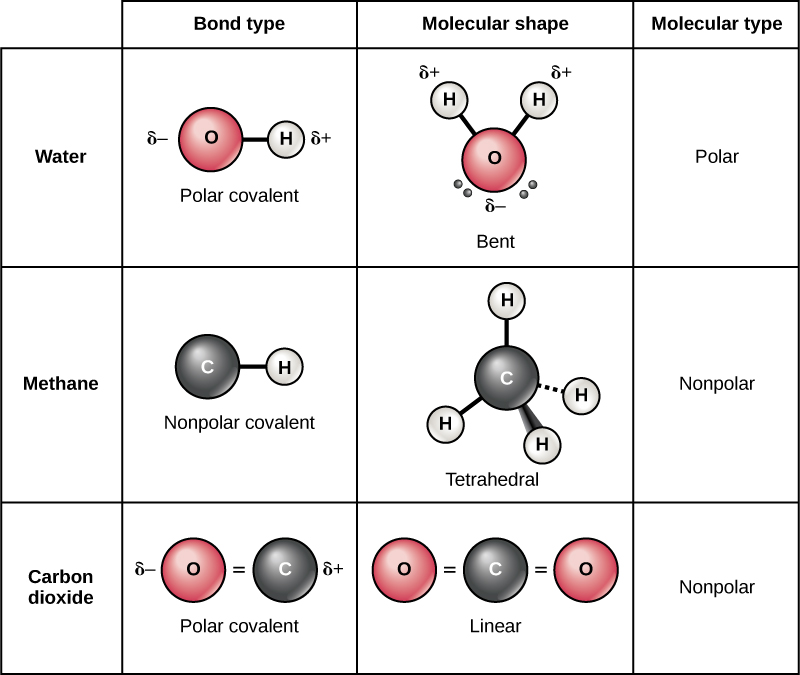
Difference Between Polar and Nonpolar Molecules Definition, Formation, Properties, Examples
where. μ is the dipole moment,; q is the magnitude of the charge, and; r is the distance between the charges.; The dipole moment acts in the direction of the vector quantity. An example of a polar molecule i s H 2 O.Bec ause of the lone pair on oxygen, the structure of H 2 O is bent, which means it is not symmetric. The vectors do not cancel each other out, making the molecule polar.

Hem apolar hem de polar kovalent bağ nasıl anlaşılır örnekle açıklar mısınız
Atom oksigen dalam molekul air memiliki elektronegatifitas yang lebih besar dari pada atom hydrogen yang terikat secara kovalen, menghasilkan pergeseran dipol di mana ikatan tersebut berbobot positif pada ujung hydrogen. Baca juga: Menentukan Kadar Masing-Masing Unsur dalam Senyawa Aspirin C9H8O4
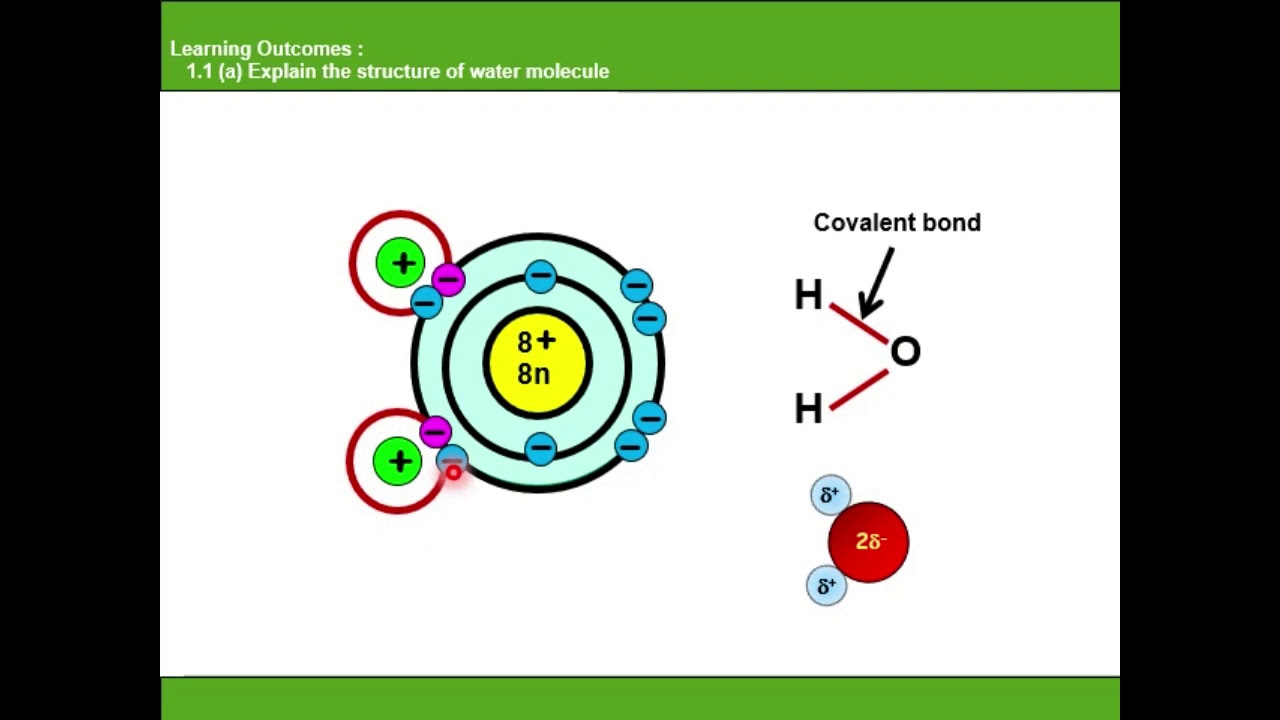
Kenapa molekul air bersifat polar bahagian 1 YouTube
3. Add all of the bonds. Use the octet rule to determine the number and type of bonds present. Each atom'svalence shell should contain 8 electrons for the molecule to be stable. Some atoms may be double or triple bonded to achieve this. [3] In a water molecule, add a single bond from the oxygen to both hydrogens.
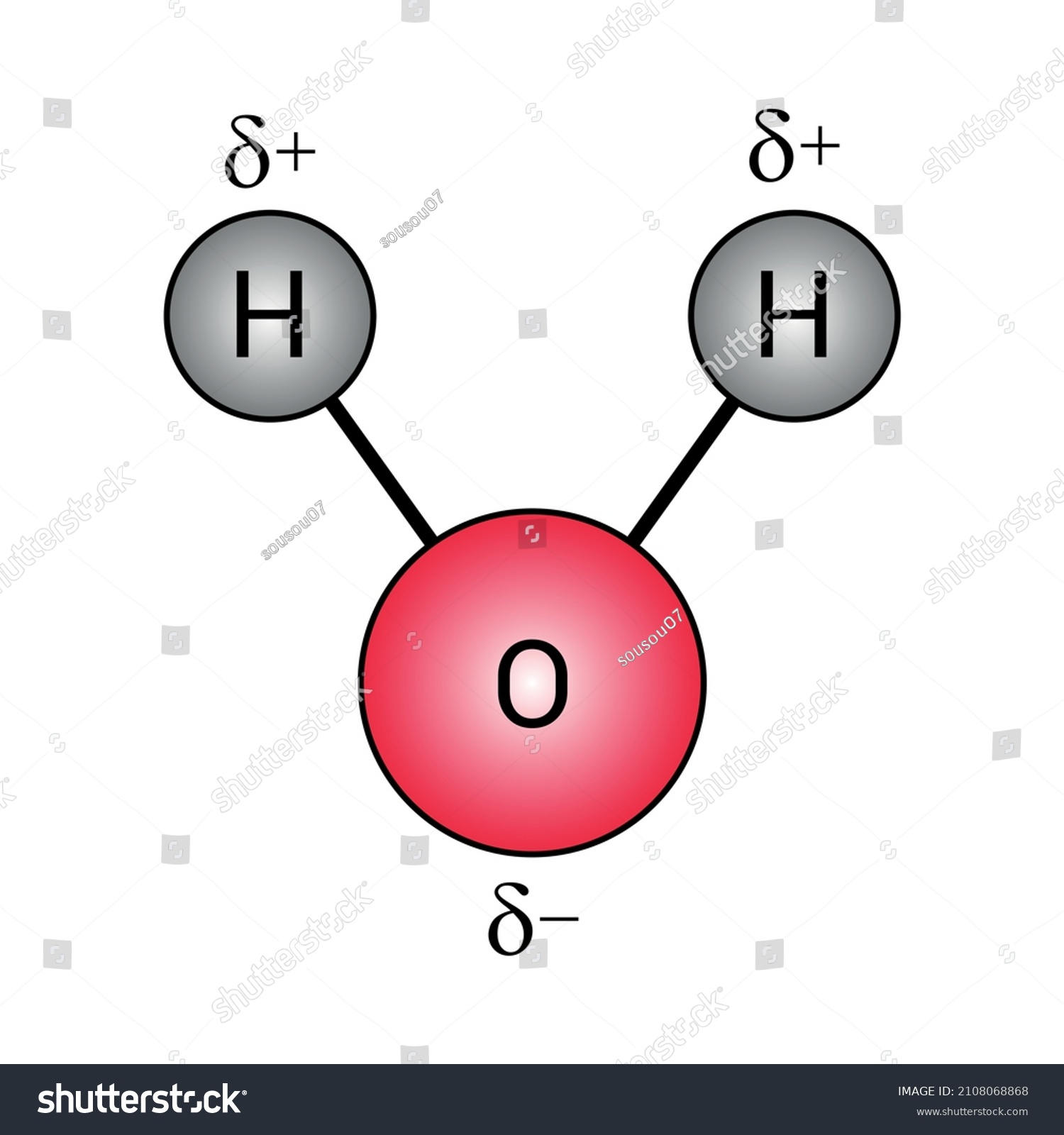
Polar Covalent Bonds Water Molecules H2o Stock Vector (Royalty Free) 2108068868
Updated on January 20, 2020 A polar molecule is a molecule containing polar bonds where the sum of all the bond's dipole moments is not zero. Polar bonds form when there is a difference between the electronegativity values of the atoms participating in a bond.
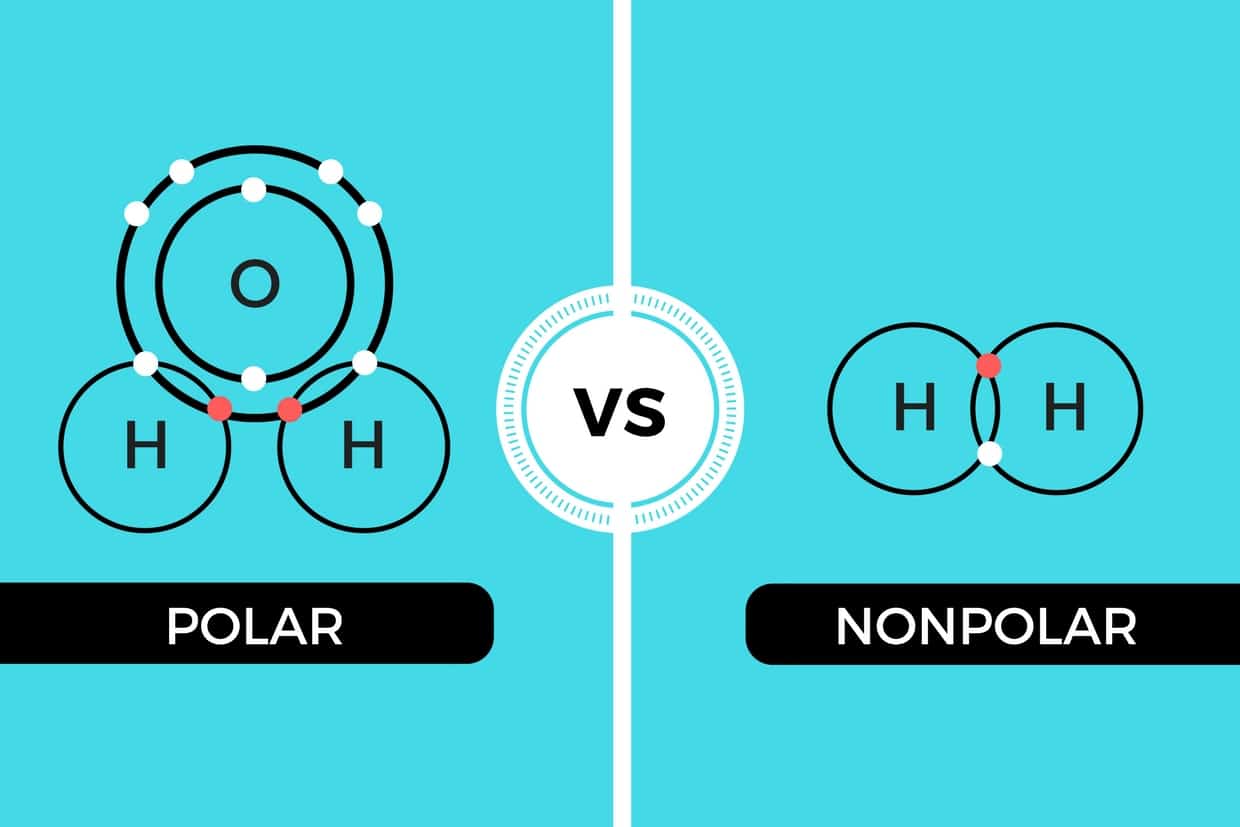
Polar and Nonpolar Covalent Bonds Characteristics & Differences Still Education
Molekul polar adalah molekul dengan ikatan polar yang dipolnya tidak dibatalkan. Artinya, mereka adalah molekul yang menghadirkan momen dipol permanen, menghasilkan perbedaan muatan listrik pada ikatan molekul. Contoh paling umum dari molekul polar adalah air (H 2 O).

Polare und unpolare Moleküle Free Press
Molekul polar dapat berinteraksi secara hidrofilik (menarik air) atau hidrofobik (menolak air) tergantung pada sifat dan susunan atom penyusunnya. Molekul polar dapat mengalami interaksi kuat antara satu sama lain, seperti gaya tarik Van der Waals, gaya dipol-dipol, dan ikatan hidrogen. Contoh molekul polar termasuk air (H₂O), amonia (NH₃.

Bentuk molekul 10 SMA (Menentukan kepolaran senyawa dari bentuk molekul) YouTube
Polar materials tend to be more attracted to and are more soluble in polar solvents. Nonpolar materials tends to be attracted to and are more soluble in nonpolar materials. Polar molecules are those that possess regions of positive and negative charge. Water is an example of a polar material. The type of bonds it has, when coupled with its.
:max_bytes(150000):strip_icc()/H20-58e1ef103df78c5162595632.jpg)
Why Is Water a Polar Molecule?
Introduction to Molecular Polarity. You have already seen that covalent bonds are polar when they link two different atoms. In a polar bond, one atom is positively charged and the other is negatively charged. A molecule (or polyatomic ion) is polar when one side of the molecule is more positive (or more negative) than the other.