
Difference Between Linear and Bent Molecules Compare the Difference Between Similar Terms
Linear combinations of atomic orbitals (LCAO) can be used to estimate the molecular orbitals that are formed upon bonding between the molecule's constituent atoms. Similar to an atomic orbital, a Schrödinger equation, which describes the behavior of an electron, can be constructed for a molecular orbital as well.
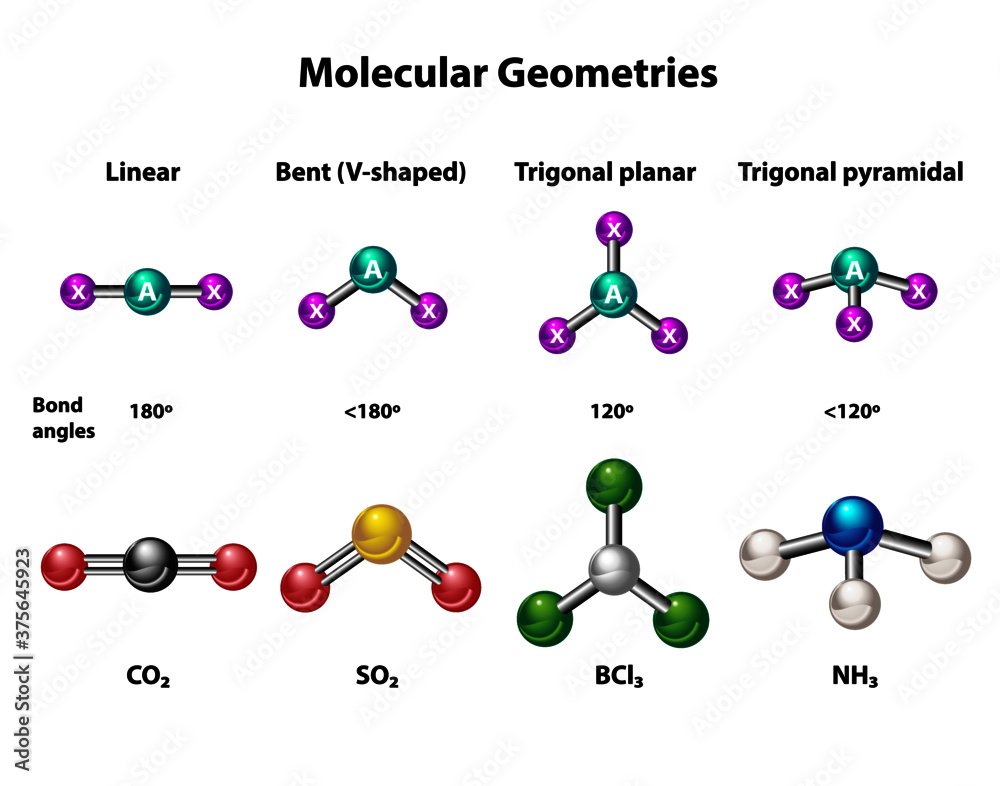
Molecular geometries in linear, bent, trigonal planar and pyramidal structures. Models and
Suatu molekul dikatakan linear jika atom-atom yang menyusun molekul tersebut berada dalam suatu garis lurus. Contohnya, BeCl2 dan CO2 . Sudut yang dibentuk oleh ikatan antara dua atom melalui atom pusat sebesar 180°. Trigonal Planar.

Image result for organic chemistry tetrahedral trigonal planar linear Molecular geometry
Bentuk linear. Suatu molekul dikatakan linear jika atom-atom yang menyusun molekul tersebut berada dalam suatu garis lurus. Contohnya, BeCl2, dan CO2. Sudut yang dibentuk oleh ikatan antara dua atom melalui atom pusat sebesar 180°. Trigonal planar.
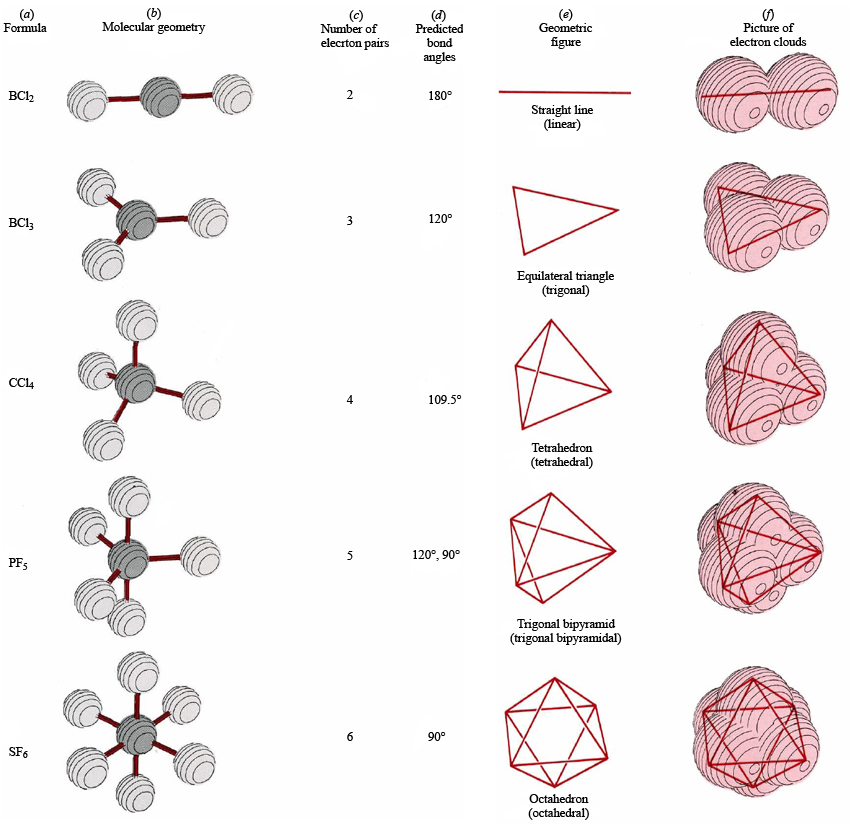
How can I find the molecular geometry of a compound? Socratic
Molecule Shapes - PhET Interactive Simulations

Bentuk Molekul Tipe dan Teori VSEPR, Domain Elektron dan Hibridasi Quipper Blog
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance.
46 Gambar 3D Bentuk Molekul Senyawa Kimia Materi Kimia
Atomic force microscopy (AFM) image of a PTCDA molecule, in which the five six-carbon rings are visible. A scanning tunneling microscopy image of pentacene molecules, which consist of linear chains of five carbon rings. AFM image of 1,5,9-trioxo-13-azatriangulene and its chemical structure. A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds.

Bentuk Molekul De Project
Atoms consist of a single nucleus with a positive charge surrounded by a cloud of negatively charged electrons.When atoms approach one another closely, the electron clouds interact with each other and with the nuclei. If this interaction is such that the total energy of the system is lowered, then the atoms bond together to form a molecule. Thus, from a structural point of view, a molecule.
Pengertian Molekul, Rumus, Model, Tipe, Teori, Rumus, Contoh
Sudut yang dibentuk oleh ikatan dua atom melalui atom pusat adalah 180 derajat. Contoh molekul linear adalah BeCl2 dan CO2. 2. Trigonal Planar. Molekul dikatakan memiliki bentuk trigonal planar apabila memiliki empat buah atom dan seluruhnya berada pada bidang yang sama. Atom pusat dikelilingi tiga atom lain yang letaknya di sudut-sudut segitiga.

Bentuk Molekul Linear YouTube
Hence, Beryllium Chloride will have a linear shape or we can say its molecular geometry is Linear. The linear geometry of Beryllium Chloride leads to the bond angle (Cl-Be-Cl) of 180° to minimize bond pair-bond pair repulsions. If the bond angle is either greater than or lower than 180°, then bond pair-bond pair repulsion will not be minimum.

Download Gambar Bentuk Molekul dan Cara Memanfaatkannya Urip dot Info
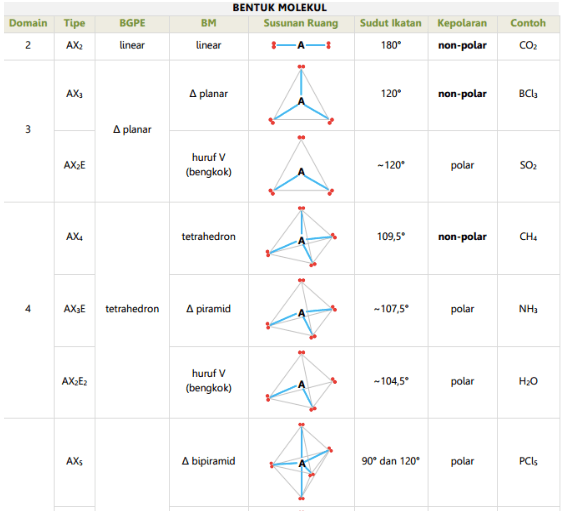
Ada 11 bentuk molekul berdasarkan teori ini, antara lain: linear (AX2), segitiga planar (AX3), segiempat piramida (AX5E), T-shape (Ax3E2), dll. Teori Hibridisasi Terakhir, ada teori hibridisasi yang menyatakan bahwa suatu ikatan molekul terjadi akibat terbentuknya orbital hibrida pada sub orbital atomnya.
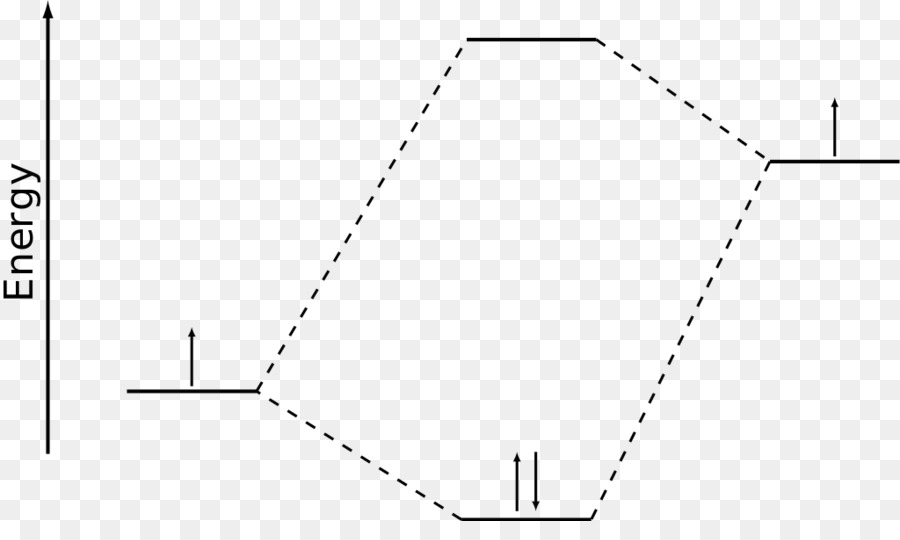
Orbital Molekul, Linear Combination Of Atomic Orbital, Orbital Atom gambar png
This is the hydrogen molecule ion, which consists of two nuclei of charge +1, and a single electron shared between them. As two H nuclei move toward each other, the 1 s atomic orbitals of the isolated atoms gradually merge into a new molecular orbital in which the greatest electron density falls between the two nuclei.
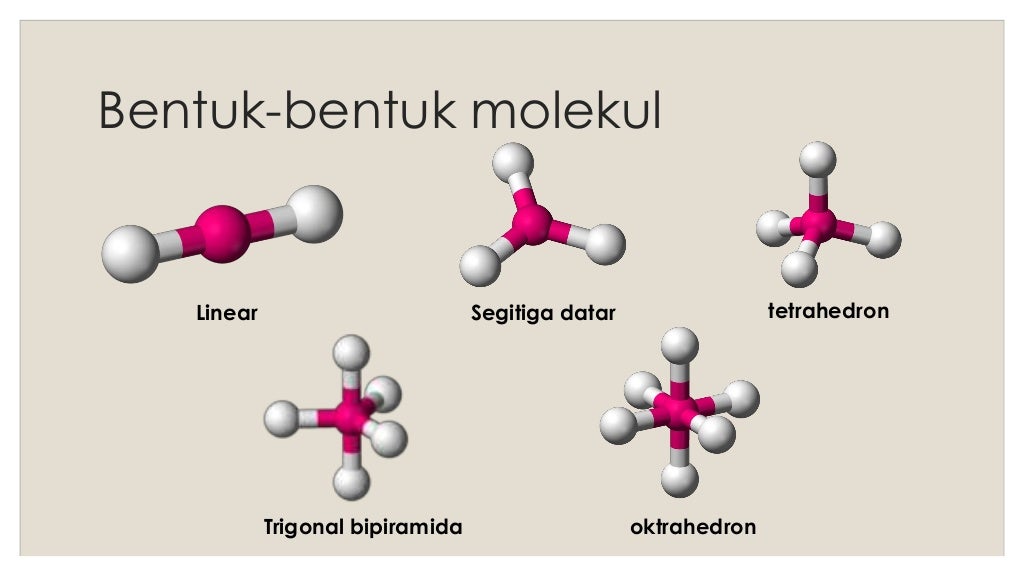
Bentuk molekul
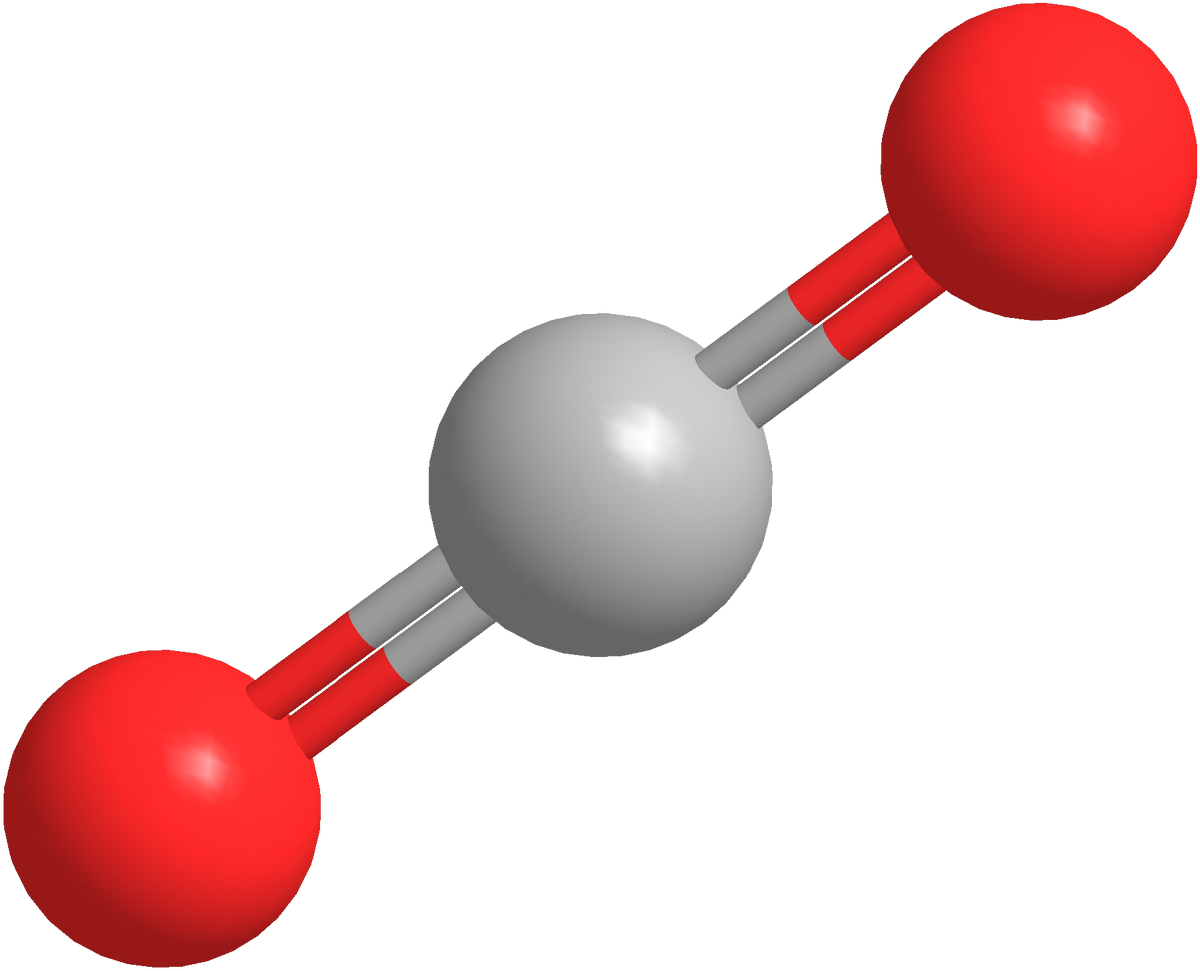
A linear molecule is one in which the atoms are arranged in a straight line (less than a 180° angle). The sp hybridization occurs at the central atom of molecules with linear electron-pair geometries. Carbon dioxide (O=C=O) and beryllium hydride BeH 2 are examples of linear electron pairs and molecular geometry.

Tabel Bentuk Molekul Berdasarkan Pei Dan Peb Berbagi Bentuk Penting
From Figure 6.3.3 we see that with two bonding pairs, the molecular geometry that minimizes repulsions in BeH 2 is linear. 5. In Section 6.2 we showed that the atomic orbitals of the Be atom were hybridized to for sp bonds, which gave BeH 2 its linear shape. AX 2: CO 2. 1. The central atom, carbon, contributes four valence electrons, and each.

Molekul Linear YouTube
Since Hückel theory is a special consideration of molecular orbital theory, the molecular orbitals | ψi can be described as a linear combination of the 2pz atomic orbitals ϕ at carbon with their corresponding {ci} coefficients: | ψi = c1 | ϕ1 + c2 | ϕ2 . This equation is substituted in the Schrödinger equation: ˆH | ψi = Ei | ψi .

MacamMacam Bentuk Molekul
5.3: Linear Molecules. Linear molecules belong to the axial rotation group. Their symmetry is intermediate in complexity between nonlinear molecules and atoms. For linear molecules, the symmetry of the electrostatic potential provided by the nuclei and the other electrons is described by either the C∞n or D∞h C ∞ n or D ∞ h group.
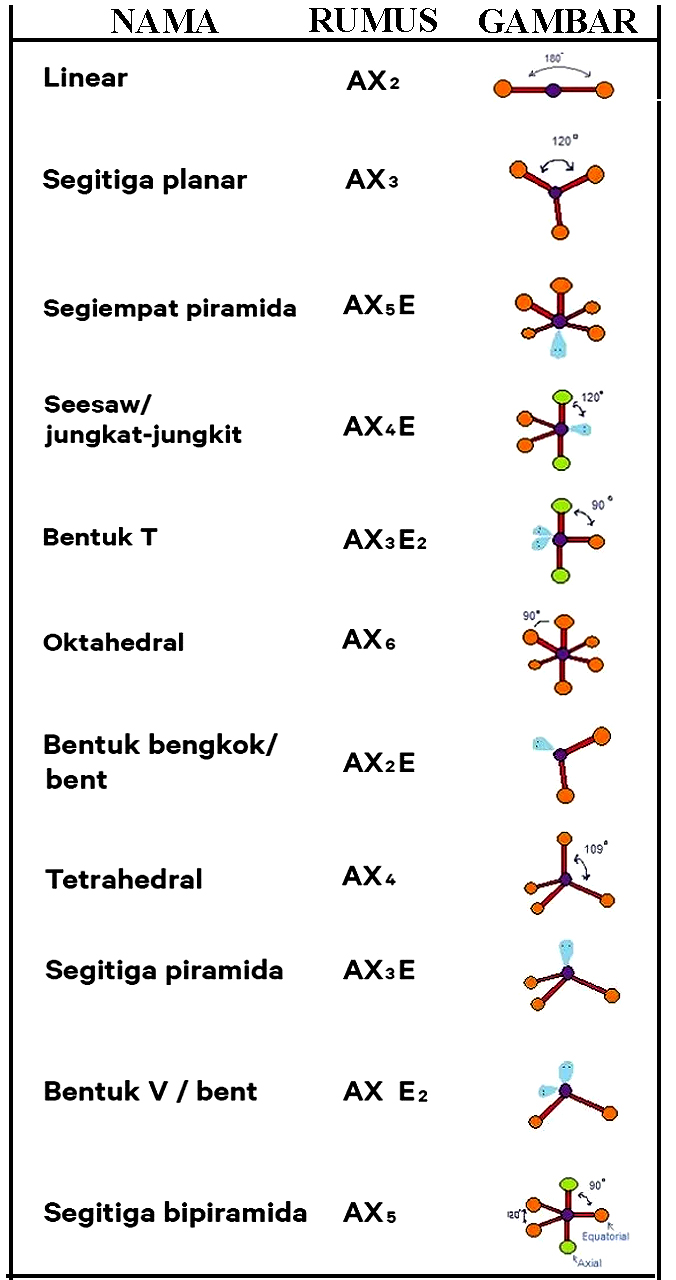
Bentuk Bentuk Molekul Menurut Teori Domain Elektron dan Hibridisasi Anto Tunggal
n: jumlah PEI dalam molekul. E: pasangan elektron bebas (PEB) m: jumlah PEB dalam molekul. Ada 11 bentuk molekul berdasarkan teori domain elektron, yaitu linear, segitiga planar, segiempat piramida, seesaw atau jungkat-jungkit, bentuk T, oktahedral, bentuk bengkok, tetrahedral, segitiga piramida, bentuk V, dan segitiga bipiramida.