
Atomic Model Rutherford, Bohr, Dalton, Thomson Atomic Model
From the era of ancient Greek philosophy to modern quantum mechanics, the atomic theory has had multiple updates, each of which was quite revolutionary for its time.. Erwin Schrödinger came up with the quantum mechanical model of an atom. In this model, the electrons do not revolve around the nucleus in circular orbits, but rather as.
Modern Atomic Model SPM Chemistry
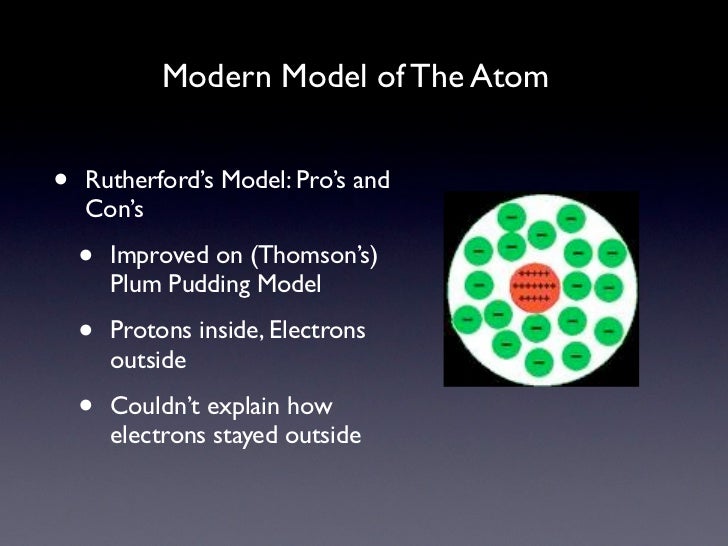
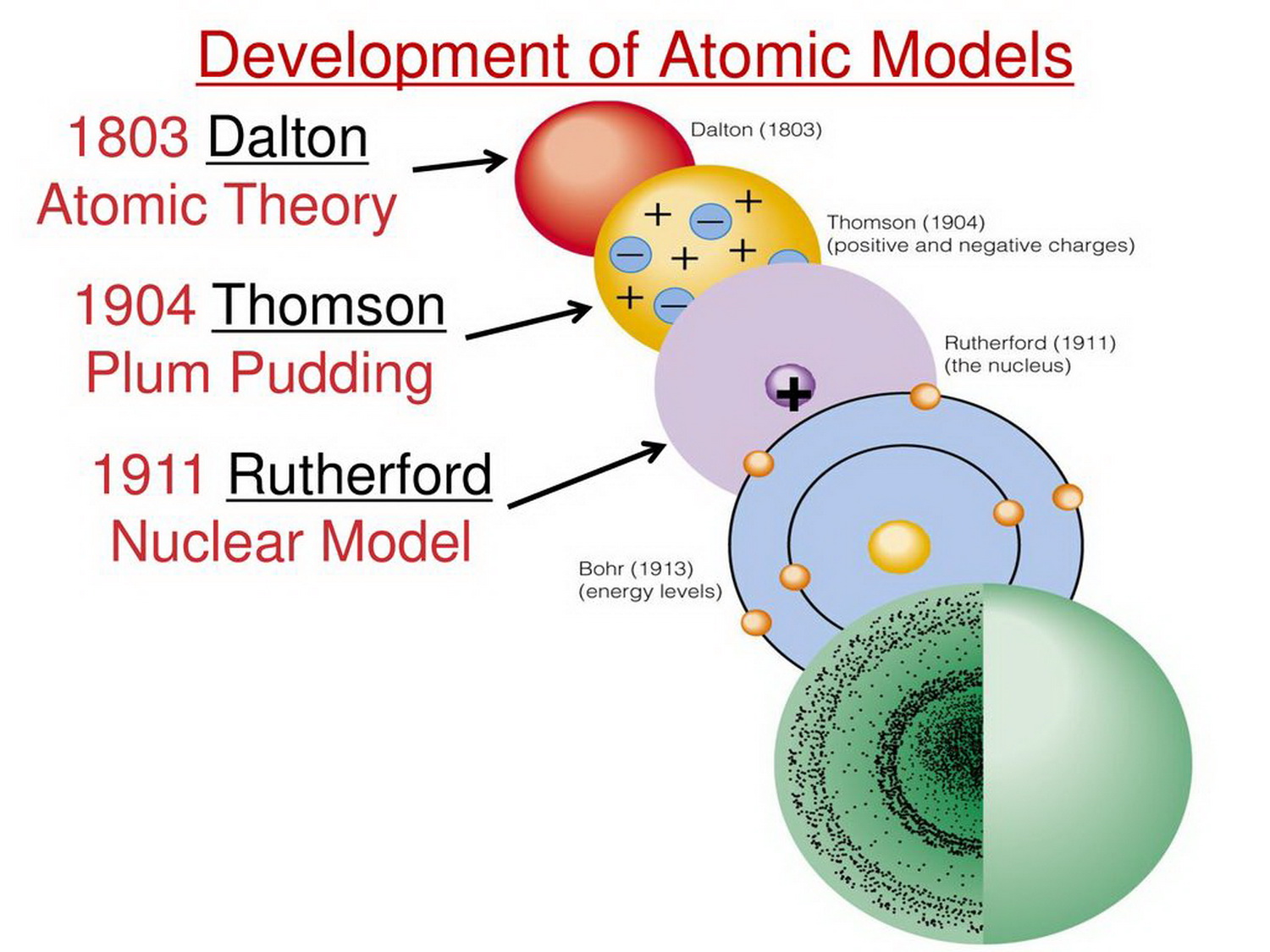
Rutherford model, description of the structure of atoms proposed (1911) by the New Zealand-born physicist Ernest Rutherford. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called electrons, circulate at some.

Atomic Structure & The Changing Models of Atom
This graphic takes a look at the key models proposed for the atom, and how they changed over time. Though our graphic starts in the 1800s, the idea of atoms was around long before. In fact, we have to go all the way back to Ancient Greece to find its genesis. The word 'atom' actually comes from Ancient Greek and roughly translates as.

The Modern Atomic Model
In general, the Bohr model encapsulates the modern understanding of the atom. This model is frequently depicted with a central atomic nucleus and oval lines representing electron orbits. The current model of the atom is the "quantum mechanical model" or the "electron cloud model" , which was developed in the 1920s and early 1930s by a.

An Introduction to the Evolution of the Modern Model of the Atom
The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. As experiments revealed more about subatomic particles, atomic models evolved from Thomson's "plum pudding model," to Rutherford's nuclear model, then to Niels Bohr's planetary model, and eventually to the currently-accepted quantum-mechanical model.
Modern Model of the Atom
To a certain extent modern atomic theory has bridged the gap between atomistic and holistic thought. Atomism - Modern Theory, Particles, Structure: With the development of a scientific atomic theory, the general philosophical problems gradually disappeared into the background. All attention is focused on the explanation of concrete phenomena.

SNC1P
Dalton's ideas proved foundational to modern atomic theory. However, one of his underlying assumptions was later shown to be incorrect. Dalton thought that atoms were the smallest units of matter − tiny, hard spheres that could not be broken down any further. This assumption persisted until experiments in physics showed that the atom was composed of even smaller particles.
Atomic Models Definition
Atom - Dalton, Bohr, Rutherford: English chemist and physicist John Dalton extended Proust's work and converted the atomic philosophy of the Greeks into a scientific theory between 1803 and 1808. His book A New System of Chemical Philosophy (Part I, 1808; Part II, 1810) was the first application of atomic theory to chemistry. It provided a physical picture of how elements combine to form.

The Modern Atomic Model Can Best Be Described as
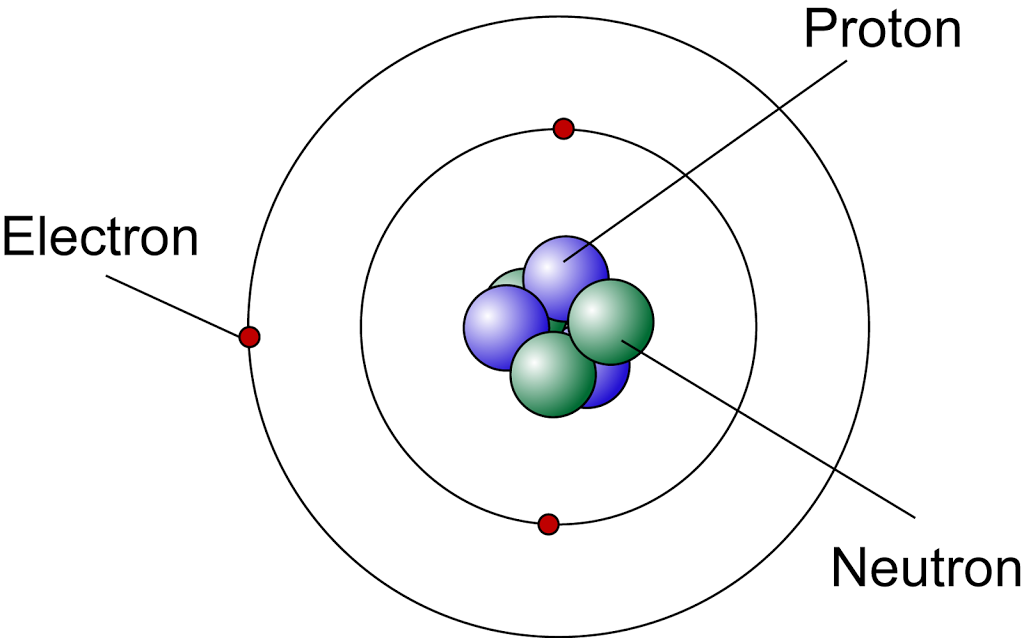
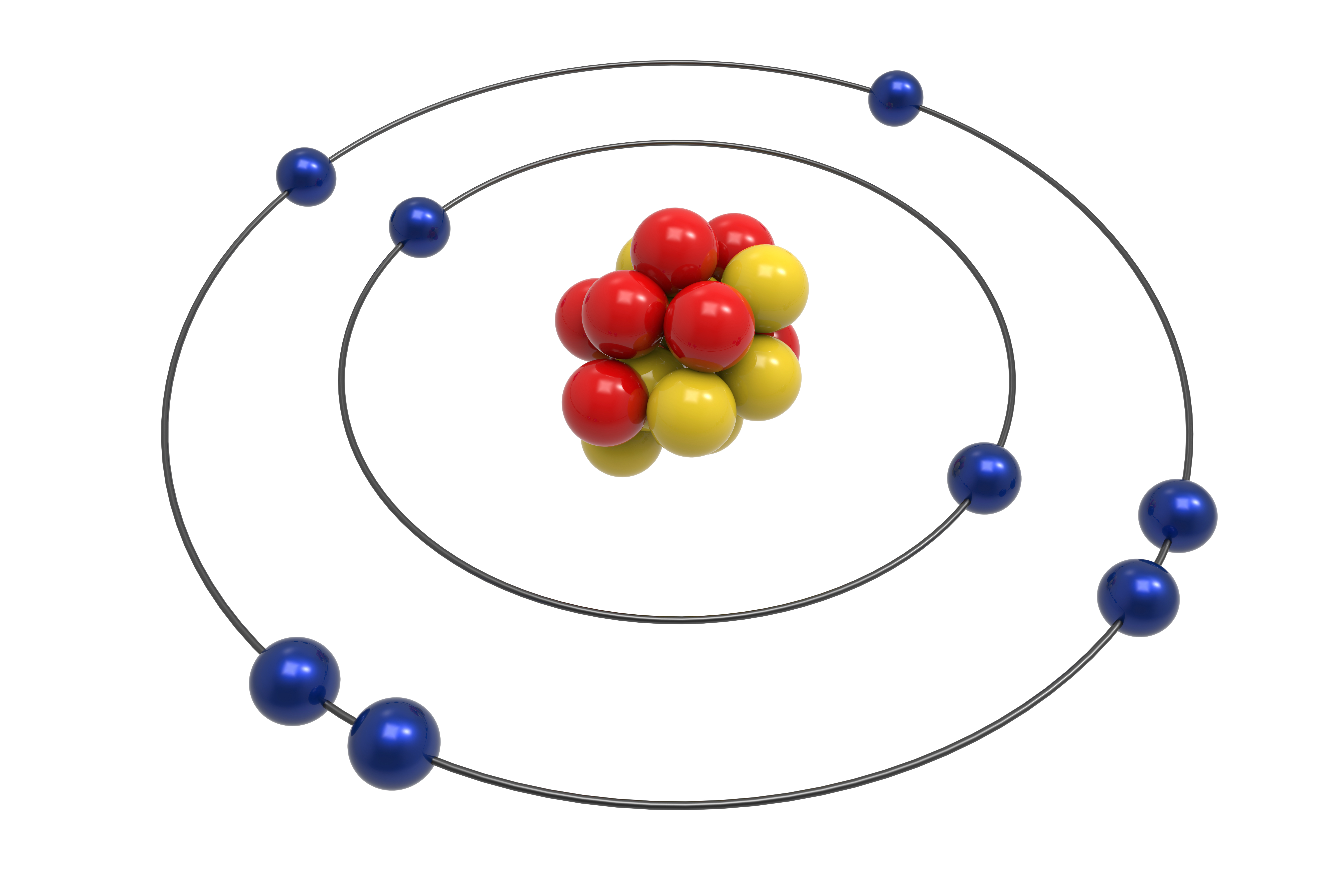
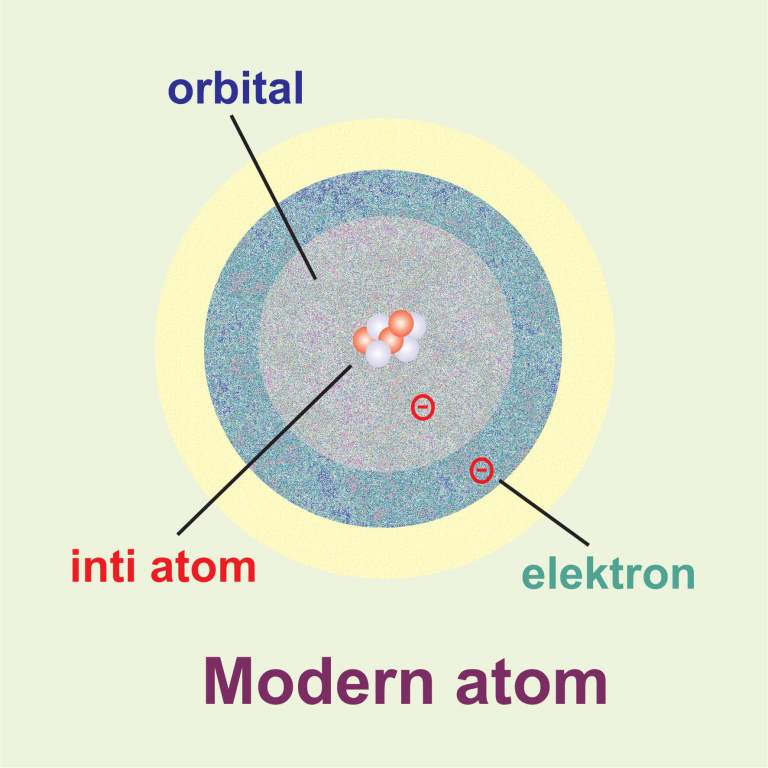
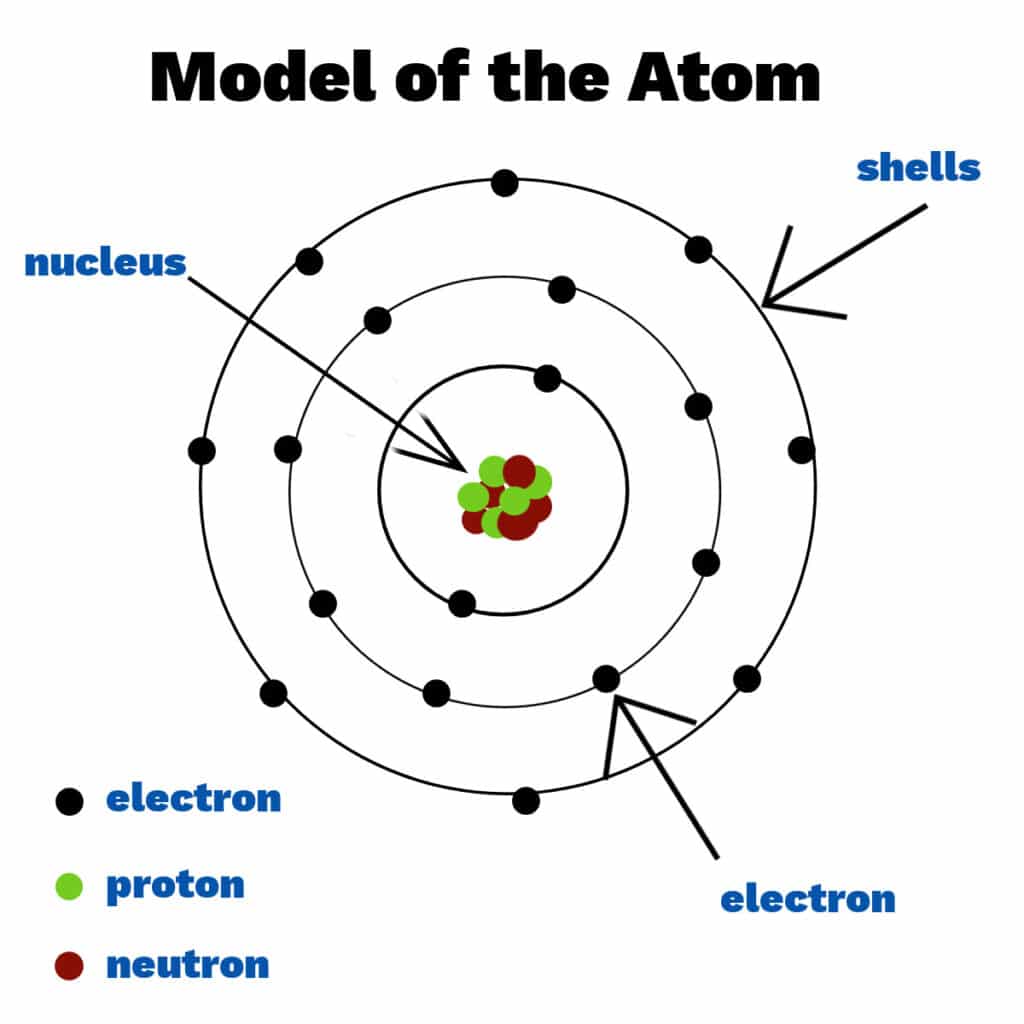
The modern atomic model represents atoms containing a nucleus of protons and neutrons and a vague gradient or cloud surrounding it containing the electrons; this is sometimes referred to as the.
Kelebihan Dan Kelemahan Atom Modern
The radius of an atom must be defined arbitrarily, such as the boundary in which the electron can be found with 95% probability. Atomic radii are typically 30-300 pm. Figure 2.3.1 2.3. 1: The structure of the nuclear atom with a central nucleus and surrounding electrons.
IB Chemistry SL & HL 2.1 Atomic Model
The current theoretical model of the atom involves a dense nucleus surrounded by a probabilistic "cloud" of electrons. Atomic theory is the scientific theory that matter is composed of particles called atoms.The concept that matter is composed of discrete particles is an ancient idea, but gained scientific credence in the 18th and 19th centuries when scientists found it could explain the.

Model Model Atom Menurut Para Ahli Seputar Model
Atomic model, in physics, a model used to describe the structure and makeup of an atom. Atomic models have gone through many changes over time, evolving as necessary to fit experimental data. For a more in-depth discussion of the history of atomic models, see atom: development of atomic theory.
PPT Modern Atomic Model and EMR PowerPoint Presentation, free download ID4823594
Bohr's model and the current model are described in Chapter 6, "The Structure of Atoms." Image used with Permission (CC BY-SA-NC). Rutherford's model of the atom is essentially the same as the modern model, except that it is now known that electrons are not uniformly distributed throughout an atom's volume.
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
Basic Model of the Atom Atomic Theory
First published in 1807, many of Dalton's hypotheses about the microscopic features of matter are still valid in modern atomic theory. Here are the postulates of Dalton's atomic theory. Matter is composed of exceedingly small particles called atoms. An atom is the smallest unit of an element that can participate in a chemical change.

Belajar 5 Teori Model Atom Dan Penjelasannya, Menarik Untuk Di Simak Ilmusaku
The Bohr model of the atom. The modern atomic model. Resources. The atom is defined as the smallest part of an element that retains the chemical properties of that element. The existence of atoms was first guessed as early as 400 BC, when Greek philosophers debated whether one could divide a substance into infinitely smaller pieces or if.
A Brief History of the Atom Top Globe News
The modern model of the atom. Today, scientists use an atomic model that has a central, positively-charged nucleus that contains: positively-charged protons close proton Subatomic particle with a.