
Massa molekul relatif (Mr) dari senyawa kalsium fosfat ( Ca3(PO4)2 ) adalah. a.365 b.310 c
15.9994. 3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in (Ca3 (PO4))2: Molar Mass (g/mol) Ca (Calcium) 6 × 40.078 = 240.468. P (Phosphorus) 2 × 30.973762 = 61.947524.

a, b The crystal structures of βCa3(PO4)2 and βCa3(VO4)2 viewed along... Download Scientific
1. What is the molecular mass of Ca3(PO4)2? (a) 310 _(b) 294 (c) 278 (d) 320 2. How will you classify lodized salt? (a) Element (b) Compound (c) mixture (d) none 3. Write the molecular mass of C12H22011? (a) 340 _(b) 342 (c) 252 (d) 100 4. What is the molecular mass of CaCO3? (a) 100 (b) 48 (C) 80 (d) 108 5. The molecular mass of KClO3? (a)122.

Solved 21. Calculate the molar mass of Ca3(PO4)2. A) 87.05
Calculate the molar mass of Ca3(PO4)2 in grams per mole or search for a chemical formula or substance. Molecular weight of Ca3(PO4)2. Ca3(PO4)2 molecular weight. Molar mass of Ca3(PO4)2 = 310.176722 g/mol. This compound is also known as Calcium Phosphate. Convert grams Ca3(PO4)2 to moles. or.

Molar Mass / Molecular Weight of Ca3(PO4)2 Calcium phosphate YouTube
15.9994. 3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in Ca3 (PO4)2: Molar Mass (g/mol) Ca (Calcium) 3 × 40.078 = 120.234. P (Phosphorus) 2 × 30.973762 = 61.947524.

The Relative Molecular Mass of ca3(po4)2 Brainly.in
The molar mass of Phosphorus is 30.974 g/mol. [2] The molar mass of Oxygen is 15.999 g/mol. [3] Now, to calculate the molar mass of Calcium phosphate, you just have to add the molar mass of all the individual atoms that are present in Calcium phosphate. So, Molar mass of Calcium phosphate [Ca3 (PO4)2] = (Molar mass of Calcium atom × 3.
[Solved] 1, What is the molar mass of Ca3(PO4)2? * 2, How many moles are in... Course Hero
Computing molar mass step by step. First, compute the number of each atom in Ca 3 PO 4: Ca: 3, P: 1, O: 4. Then, lookup atomic weights for each element in periodic table: Ca: 40.078, P: 30.973762, O: 15.9994. Now, compute the sum of products of number of atoms to the atomic weight: Molar mass (Ca 3 PO 4) = ∑ Count i * Weight i =.

SOLVEDCalculate the percent composition by mass of all the elements in calcium phosphate [Ca3
massa atom relatif dari senyawa kalsium fosfat (Ca3(PO4)2 adalah.(ar ca=40;p= 31;0=16) SD Matematika Bahasa Indonesia IPA Terpadu Penjaskes PPKN IPS Terpadu Seni Agama Bahasa Daerah

Massa Atom Relatif, Berikut Penjelasan, Rumus, dan Cara Menghitungnya
Jika diketahui Ar Fe = 56 dan O = 16, maka Mr Fe2O3 adalah. Hallo Rismawati R, jawaban untuk soal tersebut adalah 160 gam/mol. Untuk lebih jelasnya, yuk simak pembahasan berikut ^_^ Ar merupakan nomor massa atom relatif dari suatu unsur, sedangkan Mr (massa molekul relatif) merupakan total penjumlahan dari Ar setiap atom yang membentuk senyawa tertentu.
Ca3PO42 Reaktionsschema FHE Download Free 3D model by ntwerft [2cf9b35] Sketchfab
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. Since there is an equal number of each element in the reactants and products of Ca3 (PO4)2 = 3Ca {2+} + 2PO4 {3-}, the equation is balanced.
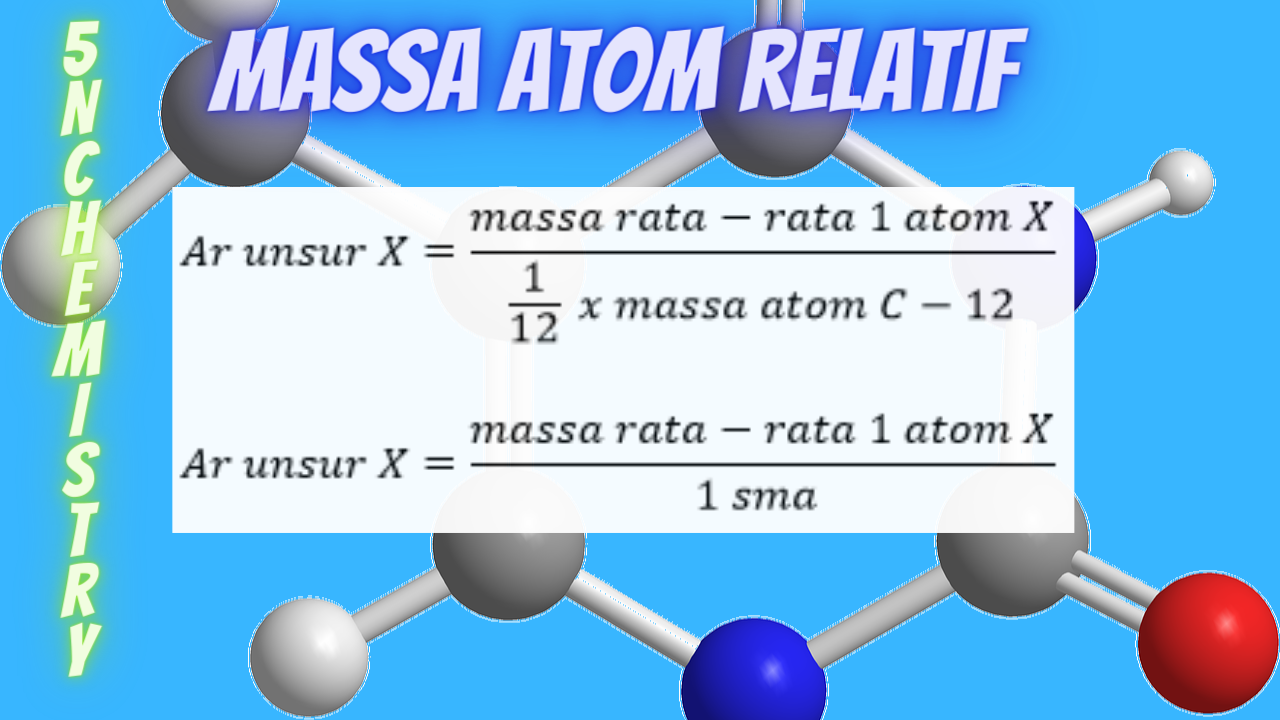
Cara Menentukan Massa Atom Relatif (Ar) Unsur 5NChemistry
Formula:Ca3(PO4)2 Enter a chemical formula to calculate molar mass,The molar mass calculator can be used in Chemical industry and medicine industry.. Formula: Ca3(PO4)2 Molar Mass: 310.18 g/mol 1g=3.22393448965117E-03 mol Percent composition (by mass):
How to Balance Ca3(PO4)2 + C = Ca3P2 + CO YouTube
Tricalcium phosphate (sometimes abbreviated TCP), more commonly known as Calcium phosphate, is a calcium salt of phosphoric acid with the chemical formula Ca 3 (PO 4) 2.It is also known as tribasic calcium phosphate and bone phosphate of lime (BPL).It is a white solid of low solubility. Most commercial samples of "tricalcium phosphate" are in fact hydroxyapatite.

The mass percent of calcium, phosphorus and oxygen in calcium phosphate, Ca3 (PO4 )2 is YouTube
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. Ca3(PO4)2 + 4 H3PO4 = 3 Ca (H2PO4)2. Reactants.
[Solved] 1, What is the molar mass of Ca3(PO4)2? * 2, How many moles are in... Course Hero
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Part 1: Calculating Molar Mass Calculate the molar mass of the compounds. 1. H2O= 2. NaCl= 3. Ca3 (PO4)2= Part 2: Determining Mass Determine the mass. 1. 3 moles Sr= 2. 4 moles H2. 3.

How to balance CaCl2+Na3PO4=Ca3(PO4)2+NaClChemical equation CaCl2+Na3PO4=Ca3(PO4)2+NaCl YouTube
The mole provides a link between an easily measured macroscopic property, bulk mass, and an extremely important fundamental property, number of atoms, molecules, and so forth. A mole of substance is that amount in which there are 6.02214076 ×1023. Figure 1.2.4: Each sample contains 6.022 10 23 atoms —1.00 mol of atoms.

Determine a massa molecular da substancia Ca3(PO4)2 YouTube
Calcium phosphate is a calcium salt of phosphoric acid with a chemical formula Ca 3 (PO 4) 2. It is also known as Calcium phosphate tribasic or Tricalcium Phosphate. Calcium phosphate appears as a white amorphous or crystalline powder that is odourless and tasteless. It is insoluble in ethanol, and acetic acid but soluble in dilute nitric acid.

Contoh Soal Massa Molekul Relatif Soal Kita
Explanation: In fact you can do this, because you do have access to a Periodic Table, and the atomic masses are printed on the Table. All scientists use the Table daily to assign molecular masses. And the atomic mass of calcium metal is 40.1 ⋅ g ⋅ mol−1, of phosphorus it is 31.00 ⋅ mol−1, of oxygen it is 15.99 ⋅ g ⋅ mol−1.