[Solved] Consider the reaction C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(g)... Course Hero
In this case, the glucose molecule loses a hydrogen ion (H+) to form C6H11O6- and water (H2O). The C6H11O6- is an anion (a negatively charged ion) because it has gained an extra electron. So, in the context of this reaction, the ions formed are OH- (from the base), H+ (from the glucose), and C6H11O6- (the product of the reaction).
Rumus Kimia Glukosa C6h12o6 Berapa Jumlah Atom C H Dan O Dalam Rumus Tersebut Rumus Kimia
Lalu, bagaimana dengan non elektrolit? Non elektrolit merupakan zat yang dalam pelarutannya gak menghasilkan ion-ion seperti pada elektrolit. Contohnya gula C6H12O6(s). C6H12O6(s)dilarutkan dengan H2O→ C6H12O6(aq) Padatan gula yang dilarutkan ke dalam air gak terurai dan gak menghasilkan ion-ion. Sehingga, gula merupakan non elektrolit.

C6H12O6+6O2>6CO2+6H2O segitiga H= 2.820 kJ C2H5OH+3O2>...
Glukosa ( C 6 H 12 O 6, berat molekul 180.18) adalah heksosa —monosakarida yang mengandung enam atom karbon. Glukosa merupakan aldehida (mengandung gugus -CHO). Lima karbon dan satu oksigennya membentuk cincin yang disebut "cincin piranosa", bentuk paling stabil untuk aldosa berkabon enam. Dalam cincin ini, tiap karbon terikat pada gugus.
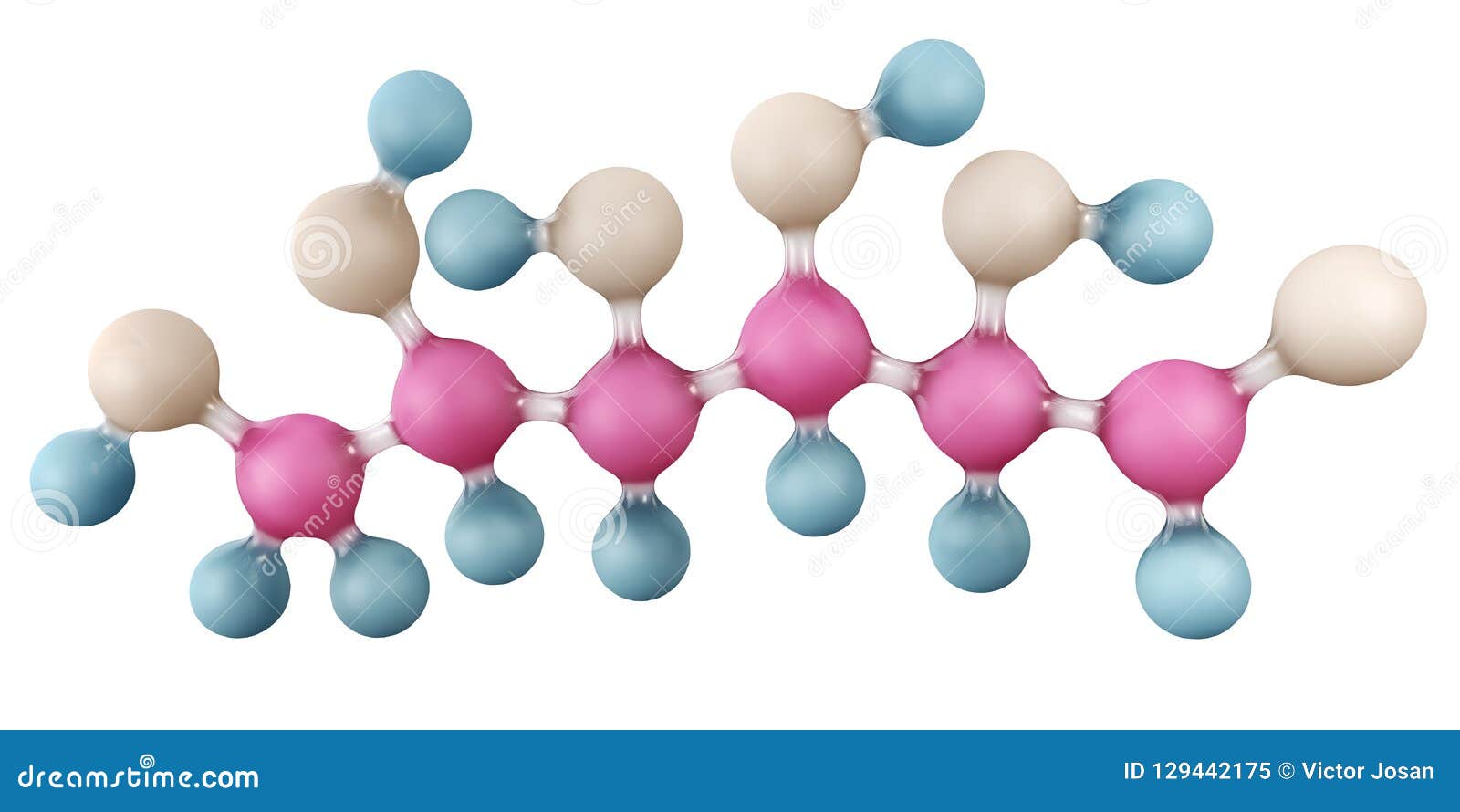
C6H12O6 Glucose Molecule Cartoon Vector 86899161
"Our study is the first randomized, placebo-controlled trial to show that a drug affecting this type of ion channel in the brain can improve depression and anhedonia in patients. Targeting this channel represents a completely different mechanism of action than any currently available antidepressant treatment," says James Murrough, MD, PhD.
[Solved] How many moles of glucose (C6H12O6) are present in 100 ml of... Course Hero
Rumus molekul senyawa merupakan rumus kimia yang menggambarkan jumlah atom dan unsur penyusun suatu senyawa, contoh : C6H12O6 , C4H8, dan lain-lain. Dalam penentuan rumus molekul, perlu ditentukan terlebih dahulu rumus empirisnya, selanjutnya dengan menggunakan data Mr senyawa, dapatlah ditentukan rumus molekulnya.

Cara mudah ikatan ion kimia SMA YouTube
Southern University of Science and Technology, School of Medicine. In 1987, two independent groups cloned a gene that encoded the potassium-ion-channel protein Shaker, enabling functional and.
Sistem Periodik Unsur Kimia Homecare24
'Ion Properties' published in 'Encyclopedia of Applied Electrochemistry' The interionic distances in crystals, of the order of 0.2-1.0 nm, are measured by diffraction of x-rays and neutrons with an accuracy of ±0.0001 nm, being fairly regularly additive in the sizes of the ions. The splitting of the interionic distances into ionic radii r I depends on the coordination number and geometry [].

C6H12O6 + O2 > 6CO2 + 6H2O + energi (glukosa) Reaksi ki...
Larutan elektrolit lemah H3PO4 0,2 M 3. Larutan non elektrolit C6H12O6 0,2 M Untuk larutan elektrolit kuat konsentrasi ion yang dihasilkan dapat ditentukan dari reaski ionisasinya 1. KOH 0,2 M Reaksi ionisasi : KOH → K+ + OH- Jumlah ion yang terbentuk : 2 Konsentrasi ion : 2 x 0,2 M = 0,4 M 2.
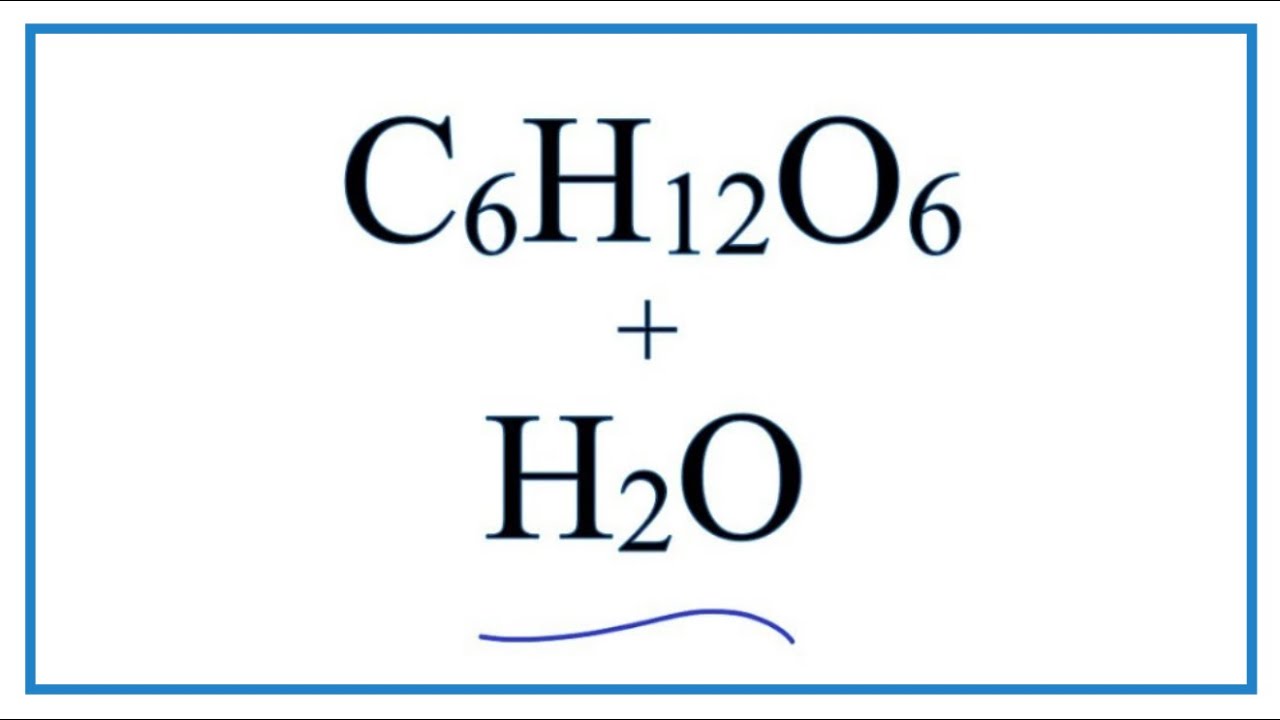
Equation for Glucose Dissolving in Water (C6H12O6 + Water) YouTube
Molar mass of C6H12O6 = 180.15588 g/mol. This compound is also known as Glucose or Fructose or Galactose. Convert grams C6H12O6 to moles. or. moles C6H12O6 to grams. Molecular weight calculation: 12.0107*6 + 1.00794*12 + 15.9994*6.

Menghitung Mr Glukosa Massa Molekul Relatif C6H12O6 Kimia SMA YouTube
Jumlah ion pada H2SO4 Adalah
- 169795. 081275747771 081275747771 05.05.2014 Kimia Sekolah Menengah Atas terjawab • terverifikasi oleh ahli Jumlah ion pada H2SO4 Adalah 1 Lihat jawaban Iklan Iklan hakimium hakimium Kelas : X Pelajaran : Kimia Kategori : Larutan Kata Kunci : elektrolit kuat, asam kuat, basa kuat, ionisasi, jumlah ion.
Cara Praktis Menghitung Jumlah Pasangan Elektron Bebas Atom Pusat dalam Molekul atau Ion
Glucose is a sugar with the molecular formula C 6 H 12 O 6.Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates.Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, the most abundant carbohydrate in the world.

Dalam 3,6 glukosa (C6H12O6) mempunyai jumlah mol sebanyak.... YouTube
Glucose is a simple sugar with six carbon atoms and one aldehyde group. This monosaccharide has a chemical formula C 6 H 12 O 6. It is also known as dextrose. It is referred to as aldohexose as it contains 6 carbon atoms and an aldehyde group. It exists in two forms, open-chain or ring structure.

Is C6H12O6 (Glucose) Ionic or Covalent/Molecular? YouTube
dimana : a = derajat ionisasi dan n= jumlah ion. contoh soal : 1) . tentukan fraksi mol larutan 36 gram glukosa (C6H12O6) dalam 90 gram H2O , jika Ar C = 12, Ar H =, 1 , dan Ar O = 16 ! diketahui : massa molar C6H12O6 : 180 gr/ mol massa C6H12O6 : 36 gr
massa molar H2O : 18 gr/mol massa H2O : 90 gr ditanya : Xt? jawab :

Total number of optical isomers of glucose molecule (C6H12O6) YouTube
Look up hexose, aldohexose, ketohexose, or inositol in Wiktionary, the free dictionary. The molecular formula C6H12O6 ( molar mass: 180.16 g/mol) may refer to: This set index page lists chemical structure articles associated with the same molecular formula. If an internal link led you here, you may wish to change the link to point directly to.
Is C6H12O6 (Glucose) an Electrolyte or NonElectrolyte? YouTube
Warning: One of the compounds in C6H12O6 + I2 = C6H12O6I2 is unrecognized. Verify 'C6H12O6I2' is entered correctly. ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another equation. Reaction Information Disclaimer. C 6 H 12 O 6 +I 2 =C 6 H 12 O 6 I 2. Reaction Type.

A sample of glucose C6H12O6 contain 20 X 10^22 atoms of carbon a How many atoms of hydrogen does
To calculate the oxidation numbers for C6H12O6, count the number of atoms, draw the lewis structure by adding bonds, assign electrons from each bond, and count the number of electrons assigned to each atom. Count Atoms Use the chemical formula, C 6 H 12 O 6, to count the number of atoms of each element.