
Isotop, isobar,dan isoton kimia SMA YouTube
Isobars, Isotopes and Isotones. Isotopes are atoms having same atomic number (Protons) but different mass number. i.e., the number of neutrons are different. Hence the atomic weights of the isotopes of an element are different. Isotopes of the same element have the same chemical properties because they have the same number and arrangement of.
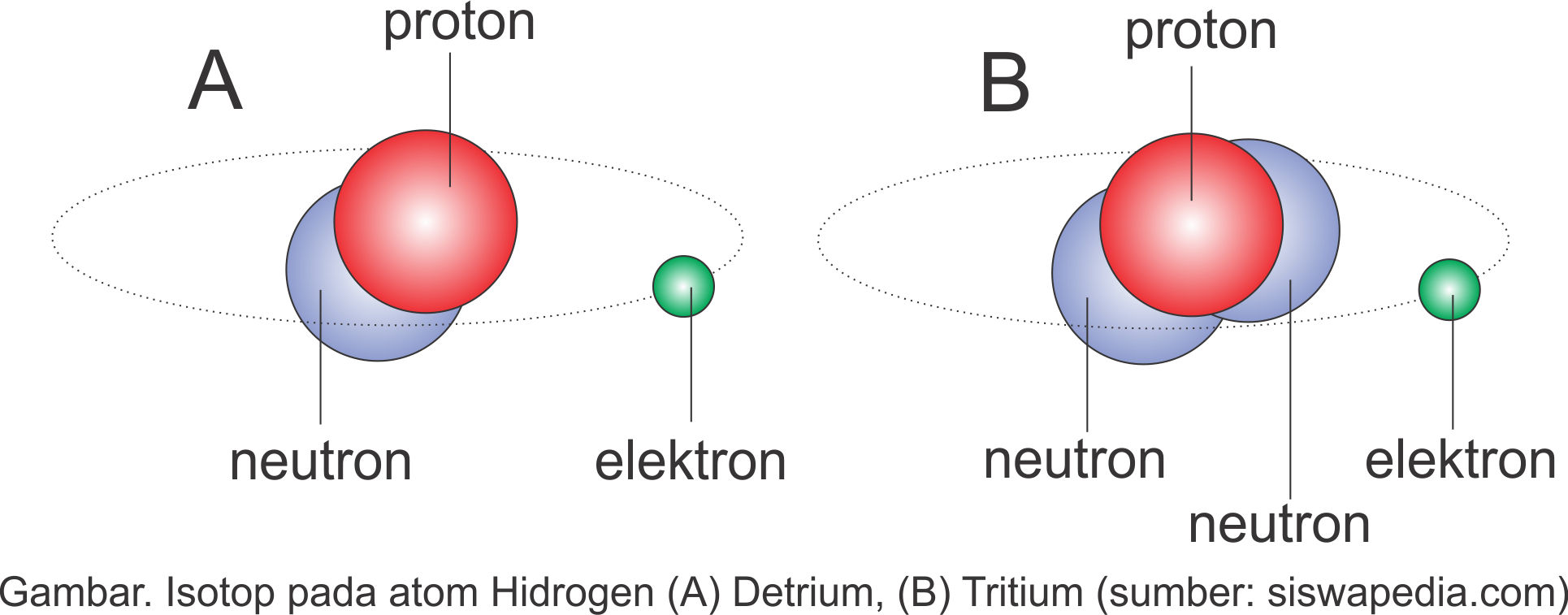
Pengertian Serta Contoh Isotop, Isobar dan Isoton Siswapedia
The terms isotopes, isobars, and isotones are used to describe the interactions between the atoms of various chemical elements. The concept of the nucleus was discovered by Rutheford in his atomic model popularly known as Rutherford's atomic model, which states that ' Protons and neutrons, which constitute almost all of the mass of the.

Isotop, Isobar, dan Isoton YouTube
Thus, the major difference between Isotopes and Isobars is that isotopes belong to the same element while isotones are different elements. Also, isotopes have similar chemical properties. However, isobars have different chemical properties. For example, 12 C, 13 C, and 14 C are the isotopes of Carbon while 26 Fe 58 and 27 Ni 58 are Isotones.

Pembahasan Soal Isotop, Isobar, dan Isoton Struktur Atom YouTube
Isotopes and Isobars. Isobar are elements that differ in chemical properties but have the same physical property. So, we can say that isobars are those elements that have a different atomic number but the same mass number. In contrast, Isotopes are those elements having the same atomic number and different mass numbers.

Isotop, Isobar, dan Isoton My Chemistry ff
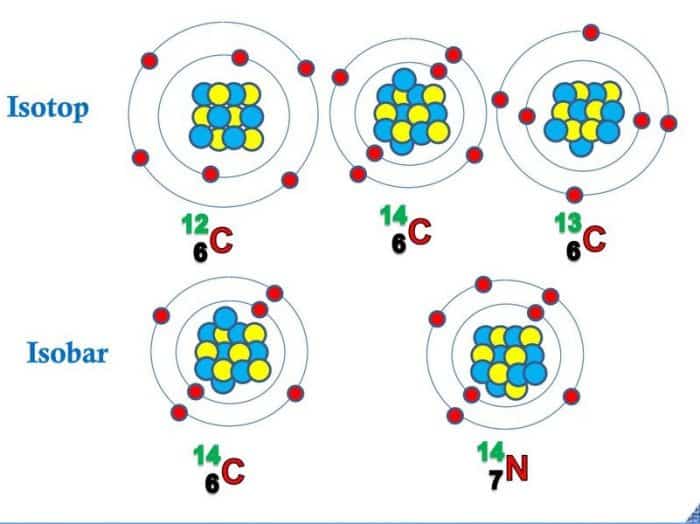
Bentuk (rupa) yang lain dari beberapa atom yang memiliki suatu kesamaan pada jumlah partikel dasar penyusunnya, meliputi isotop, isobar, isoton, dan isoelektron. 1. Isotop. Isotop ( isotope ), berasal dari kata dalam bahasa Yunani: isos (artinya: sama) dan topos (tempat) pada dasarnya menyatakan atom-atom yang memiliki tempat yang sama di dalam.

Kimia kelas x ISOTOP ISOBAR ISOTON YouTube
ISOBARS Isobar is a group of nuclides that has sum of proton and neutron are same but sum of each proton and neutron is different. • Examples : 12C6 with 12 C 7 • Examples : 12C6 with 12 C 7 5.

Chemistry Isotope, Isobar, Isotone Science Education World YouTube
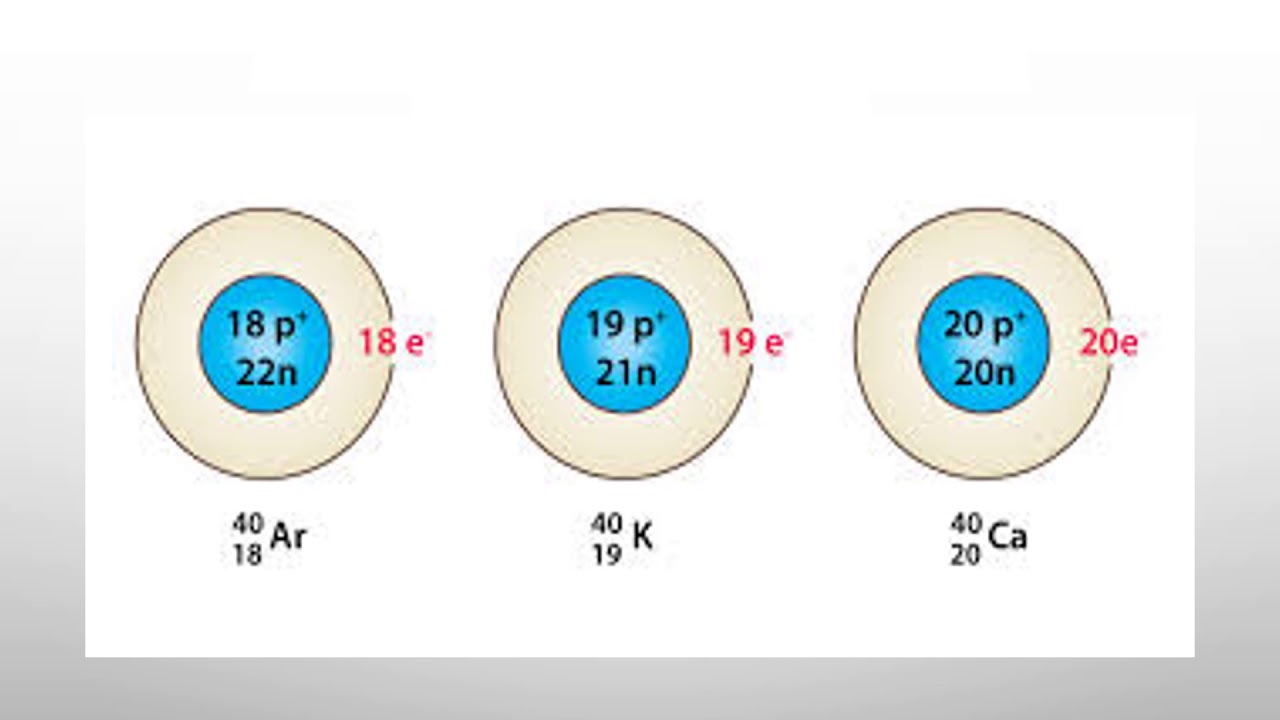
- Isobars are atoms that have the same mass number but different atomic numbers are called isobars. - The word isobar meaning 'equally heavy' is taken from the Greek isos = equal, and barys = heavy. Examples of isobars - For example, 40 Ar 18, 40 K 19, and 40 Ca 20 are isobaric atoms. - Similarly, 235 U 92, 235 Np 93, 235 Pu 94 are.
Isotope, Isobar and Isotone pptx YouTube
Memahami Definisi Isotop, Isobar dan Isoton dalam Ilmu Kimia Beserta Contoh. 27 Oktober 2023. Yusuf Abdhul Azis. Pada saat pertama kali mengenal istilah isotop, isobar, dan juga isoton ini mungkin kamu akan sedikit bingung bahkan pusing. Apalagi ada sedikit perbedaan antara isotop, isobar dan juga isoton. Meskipun begitu, dewasa ini proses.

Perbedaan Isotop, Isobar, Isoton, dan Isomer Muhyidin, SKM
As mentioned before, isotopes are atoms that have the same atomic number, but different mass numbers. Isotopes are denoted the same way as nuclides, but they are often symbolized only with the mass numbers because isotopes of the same element have the the same atomic number. Carbon, for example, has two naturally occurring isotopes, 12 6 C and.

10 Differences between isotopes and isobars DewWool
Iodine isobars are used to treat goiter. Cobalt isobars can be used to treat cancer. Frequently Asked Questions. Q1. What are isotopes and isobars? Answer. Isotopes are atoms of the same element with the same atomic number but a different mass number. Isobars are atoms of various elements that have the same mass number but a different atomic.
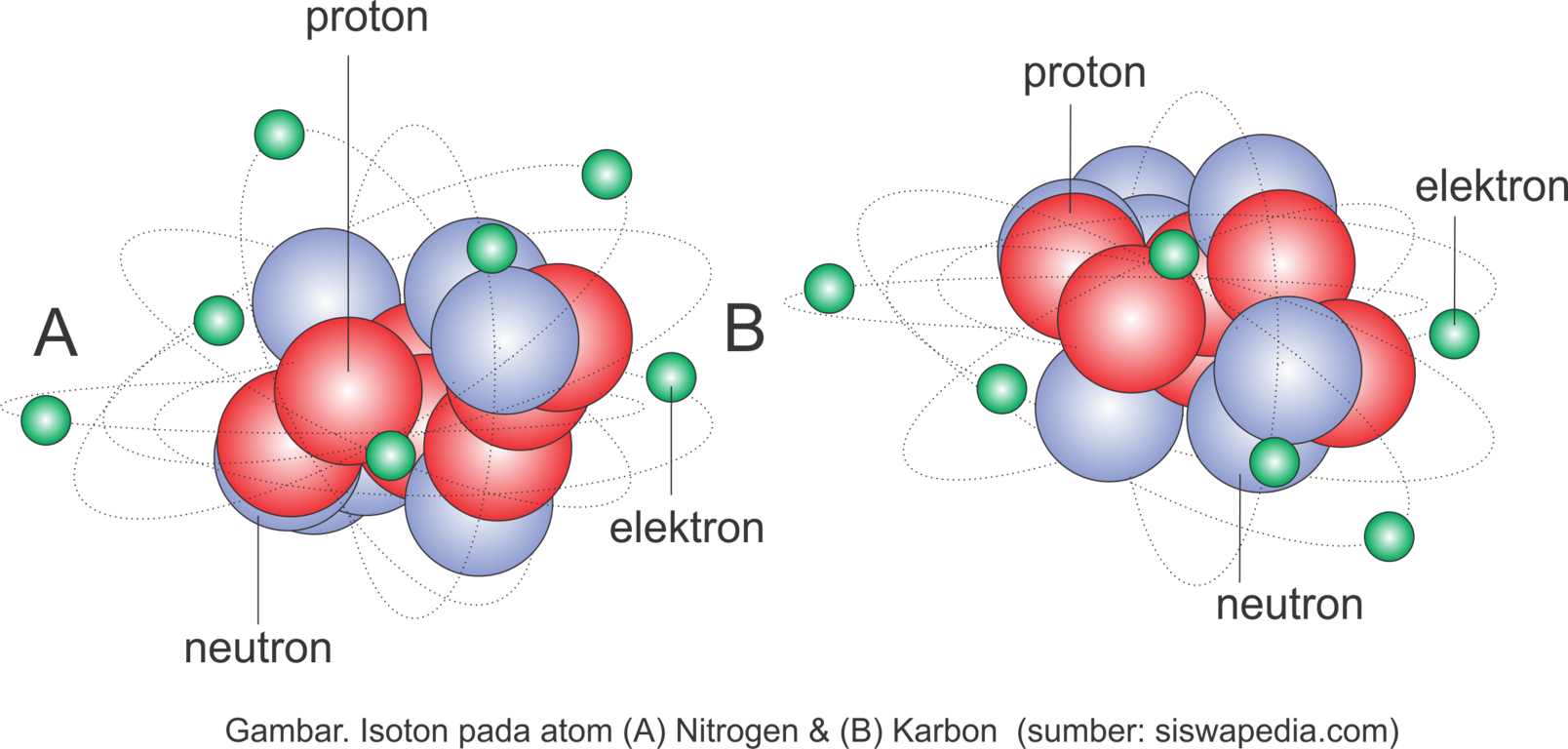
Pengertian Serta Contoh Isotop, Isobar dan Isoton Siswapedia
v. t. e. Nuclide half-lives colorcoded. Two nuclides are isotones if they have the same neutron number N, but different proton number Z. For example, boron-12 and carbon-13 nuclei both contain 7 neutrons, and so are isotones. Similarly, 36 S, 37 Cl, 38 Ar, 39 K, and 40 Ca nuclei are all isotones of 20 because they all contain 20 neutrons.

Contoh Isotop Isobar Dan Isoton cara mengatasi kaki pegal saat mau tidur
Isobars Elements having the same mass number (A) but different number of protons (Z) are isobars. Example: 40 16 S, 40 17 Cl, 40 18 Ar, 40 19 K, and 40 20 Ca. Isotones Elements having the same number of neutrons (N) but a different number of protons (Z) or mass number (A) are isotones. Example: 36 16 S, 37 17 Cl, 38 18 Ar, 39 19 K, and 40 20 Ca.

Structure of Atom (Part 1) Isotopes, isotones and Isobar Class 11, chapter 2 YouTube
Isobars. The set of elements has the same number of nucleons, where nucleons are protons or neutrons. For example, 40 Sulfur, 40 Chlorine, 40 Argon, 40 Potassium, and 40 Calcium are all isobars. Moreover, despite having the same mass number, isobars have different atomic numbers for different chemical elements.
Teori Struktur Atom Menurut Para Ahli Beserta Pengertiannya (Lengkap)
ISOTOPES, ISOBARS AND ISOTONES87 Thus Aston's Mass spectrograph not only helped in identifying the isotopes present in an element but also helped in determining the average atomic mass of a given element. 18 20 22 24 26 28 30 32 34 36 38 Neon Chlorine Mass spectrographs of Neon and Chlorine. Figure 3.2 SOLVED PROBLEM.

ISOTOP, ISOBAR, ISOTON, dan ISOELEKTRON STRUKTUR ATOM YouTube
Isobars are elements that have the same number of nucleons (sum of protons and neutrons). The series of elements with 40 Mass numbers serve as a good example; 40 16 S, 40 17 Cl, 40 18 Ar, 40 19 K, and 40 20 Ca. The nucleus of all the above-mentioned elements contain the same number of particles in the nucleus but contain varying numbers of.

Difference Between Isotopes And Isobars Understanding The Key Differences A Plus Topper
Isotopes. Isotopes are atoms of an element which have the same proton number but different nucleon numbers. Example: Hydrogen is the common example which has three isotopes. These have the same atomic number, one, but different mass numbers 1, 2, and 3. These three isotopes are commonly known as hydrogen or protium, deuterium (D) and tritium (T.