
Perhatikan tabel berikut! No Garam Hasil Reaksi Hidrolisi...
On hydrolysis of sodium carbonate, the reaction takes place between ? Q. In a solution of N aHCO3, the amphiprotic anion undergoes ionization to form H+ ion and hydrolysis to form OH−ion. HCO− 3+H2Oionization ⇋ CO2− 3 +H2O. HCO− 3+H2Oionization ⇋ H2CO3+OH−. To calculate pH of the above solutions, the suitable approximation is.
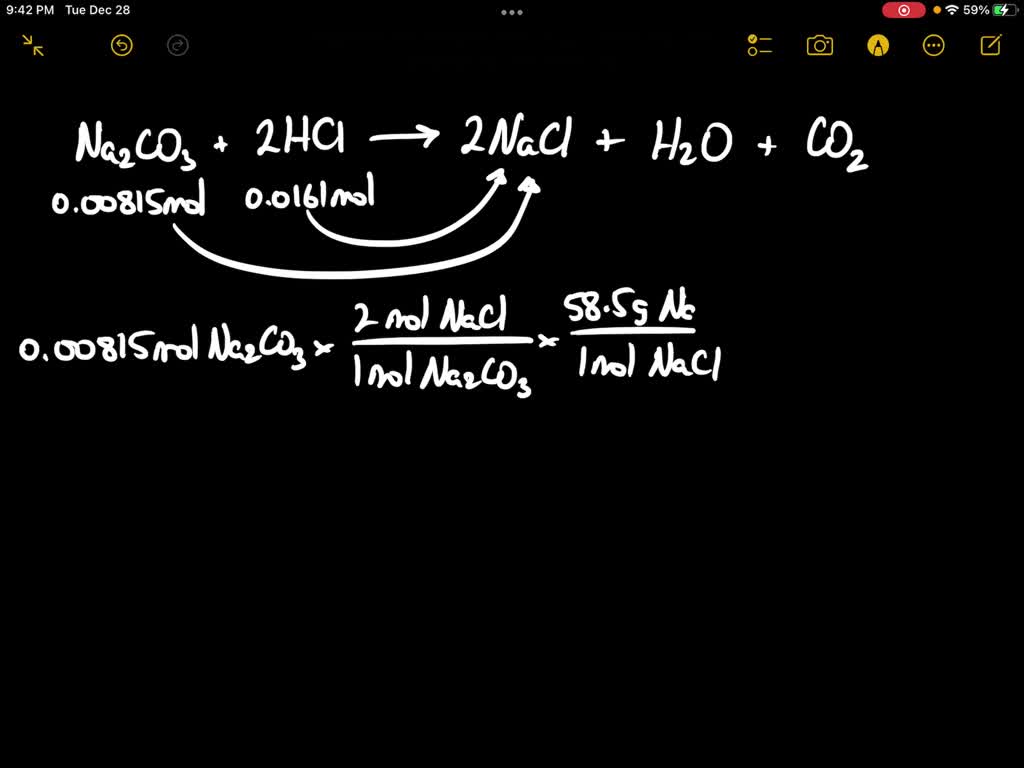
SOLVED A student reacts 0.00718 moles of sodium carbonate with 0.0136 moles of hydrochloric
El ion de amonio es el ácido conjugado de la base amoníaco, NH 3; su reacción de ionización ácida (o hidrólisis ácida) está representada por. NH4+(aq) + H2O(l) ⇌ H3O+(aq) + NH3(aq) Ka = Kw/Kb. Como el amoníaco es una base débil, Kb es medible y Ka > 0 (el ion de amonio es un ácido débil).

Hidrólisis qué es, cómo se lleva a cabo y cuál es su importancia Renovables Verdes
To tell if Na2CO3 (Sodium carbonate) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that N is a metal and CO3 is a g.

Standardization Of HCl With Na2CO3; Standardization Of NaOH With Standard HCl1/ AcidBase
Hydrolysis involving ionic compounds may be illustrated by the chemical changes occurring in an aqueous solution of the salt sodium acetate. In solution, the ionic constituents of the salt (the acetate ion and the sodium ion) separate; water molecules combine with the acetate ions to form acetic acid and hydroxide ions. Acetic acid dissociates reversibly into acetate ions and hydrogen ions.
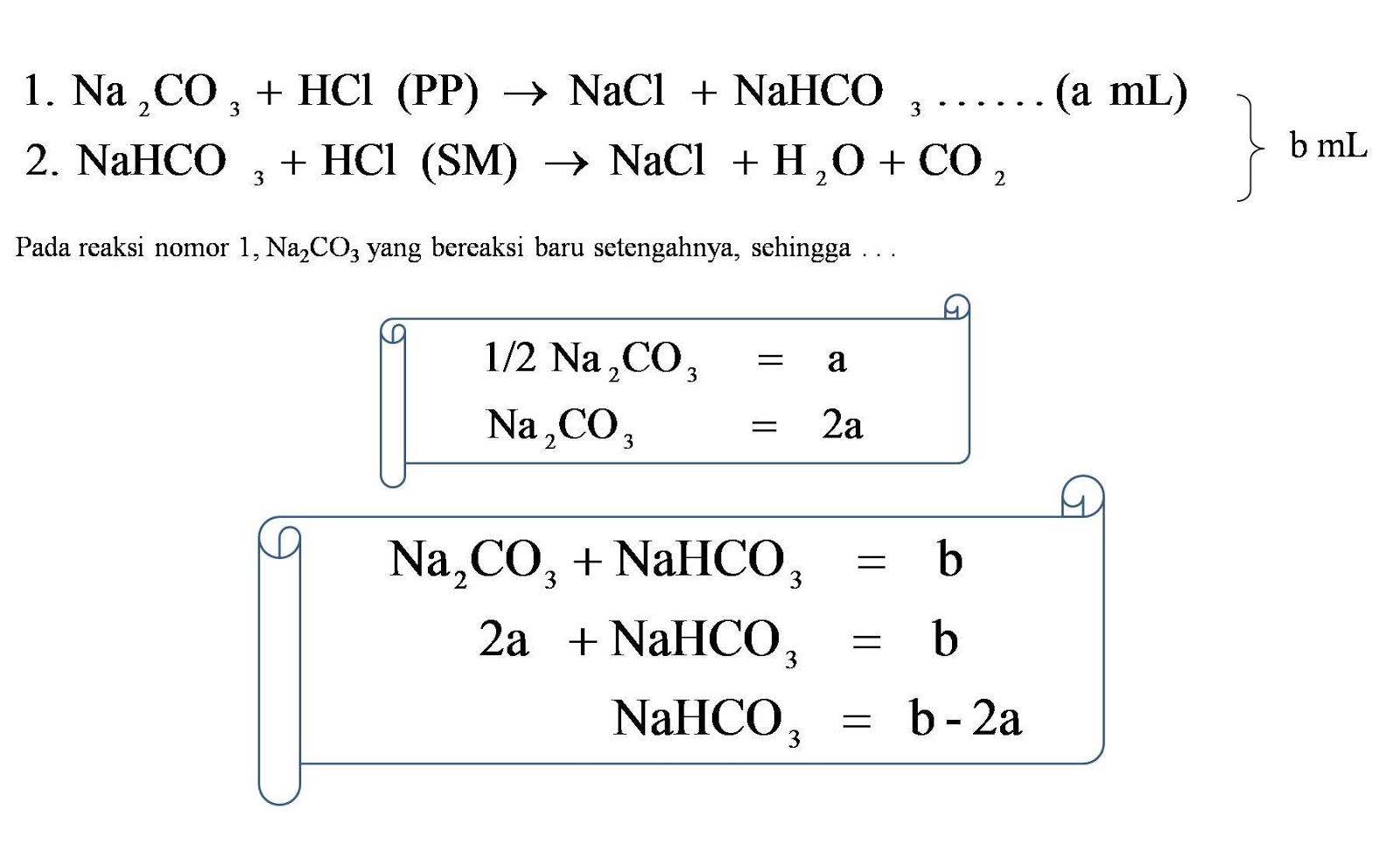
Kadar Campuran Na2CO3 dan NaHCO3 Cara Warder
Na2CO3 (s) —-> 2 Na+(aq) + CO3-2(aq) The carbonate ion is able to remove protons (H+) from water to form bicarbonate ions and hydroxide ions. Hence it is the conjugate base of the bicarbonate ion;

TEM image after mixing of a) 40mM MgCl2 and 40mM Na2CO3, b) 40mM BaCl2... Download Scientific
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. Na2CO3 + 2 HCl = 2 NaCl + H2O + CO2. Reactants.

PPT Types of Chemical Reactions PowerPoint Presentation, free download ID3726756
The ammonium ion is the conjugate acid of the base ammonia, NH 3; its acid ionization (or acid hydrolysis) reaction is represented by. NH4+(aq) + H2O(l) ⇌ H3O+(aq) + NH3(aq) Ka = Kw/Kb. Since ammonia is a weak base, Kb is measurable and Ka > 0 (ammonium ion is a weak acid). The chloride ion is the conjugate base of hydrochloric acid, and so.

Гидролиз солей презентация онлайн
El carbonato de sodio (Na2CO3) es una sal inorgánica de sodio, de metal alcalino y del ácido carbónico. También se le conoce mundialmente como ceniza de sosa. Los lagos y las actividades volcánicas enriquecieron de sodio los suelos, de los cuales se nutrieron las plantas; luego, tras un incendio, estas plantas esparcían las cenizas de carbonato.

TOMi.digital Síntesis y función biológica de los ácidos carboxílicos
Explicación sobre cómo encontrar el pH de una solución con doble hidrólisis del ácido carbónico asociado a la sal de carbonato de sodio (Na2CO3)utilizando la.

Tipos de Hidrolisis
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. 2 HCl + Na2CO3 = 2 NaCl + H2O + CO2. Reactants.

pasión evidencia Barón disociacion de nh3 en agua Coherente Centelleo es suficiente
Improve this question. This is the equation given by my textbook for hydrolysis of sodium carbonate: NaX2COX3 +2HX2O HX2COX3 +2NaX+ +2OHX− N a X 2 C O X 3 + 2 H X 2 O H X 2 C O X 3 + 2 N a X + + 2 O H X −. and it mentions that sodium ion (NaX+) ( N a X +) does not tend to combine with the hydroxide ion (OHX−) ( O H X −) and I was.

Senyawa CaCl2, Na2CO3 , dan NaCl adalah garamgaram ya...
Oleh karena itu garam dapat terbentuk dari 4 reaksi hidrolisis kimia sebagai berikut: Garam yang berasal dari asam lemah dan basa kuat bersifat netral dan memiliki pH = 7, yang artinya tidak terjadi hidrolisis. Garam dari asam kuat dan basa lemah, di mana garam ini akan memiliki pH < 7 alias bersifat asam. Garam dari asam lemah dan basa kuat.
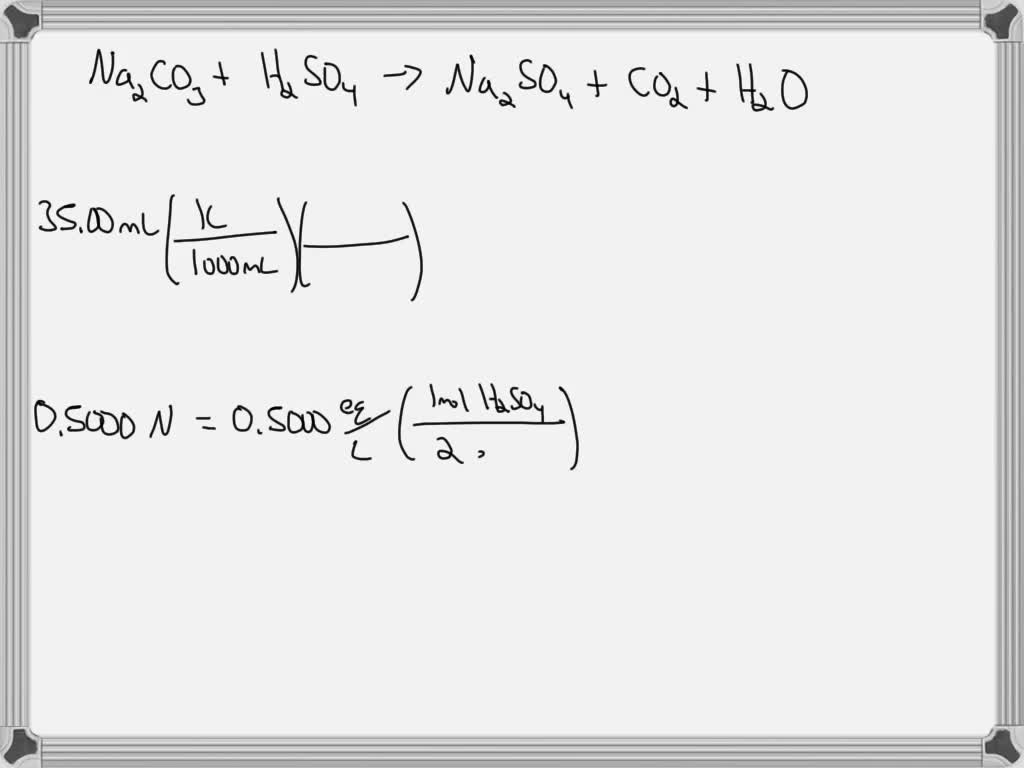
SOLVED Na2CO3 reacts with H2SO4 to give respective salt water and carbon dioxide. Calculate the
Hidrolisis - Kimia Kelas 11 - Teori, Jenis Reaksi, dan Contoh Soal. by sereliciouz & Andjar Tyassih, S.Si. Agustus 28, 2019. Dalam artikel ini akan dibahas secara detail tentang teori dan reaksi hidrolisis, jenis-jenis reaksi hidrolisis, contoh soal dan pembahasan reaksi hidrolisis, dan aplikasi reaksi hidrolisis dalam kehidupan sehari-hari.
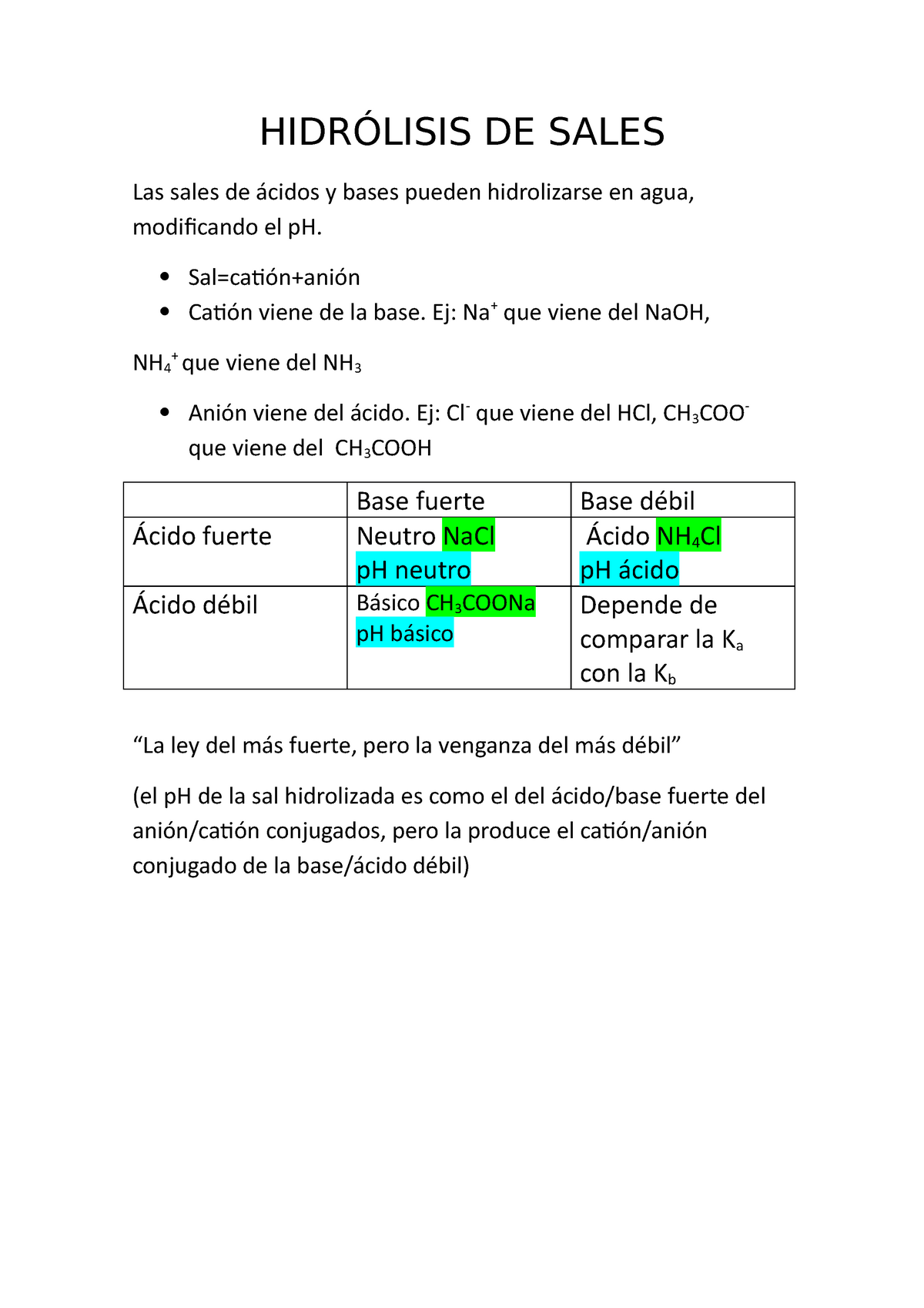
Hidrólisis DE Sales HIDRÓLISIS DE SALES Las sales de ácidos y bases pueden hidrolizarse en
Question: What are the net ionic equations for the hydrolysis of the the following:NaC2H3O2Na2CO3NH4CLZnCl2KAl (SO4)2KAl (SO4)2 for 5 & 6 there are supposed to be 2 different hydrolysis reactions occuringAlso determine if each is Ka or Kb. What are the net ionic equations for the hydrolysis of the the following: Here's the best way to solve it.

Na2CO3 + Cl2 → NaCl + NaClO + CO2↑ Na2CO3 ra NaCl
In this video we will describe the equation Na2CO3 + H2O and write what happens when Na2CO3 is dissolved in water.When Na2CO3 is dissolved in H2O (water) it.
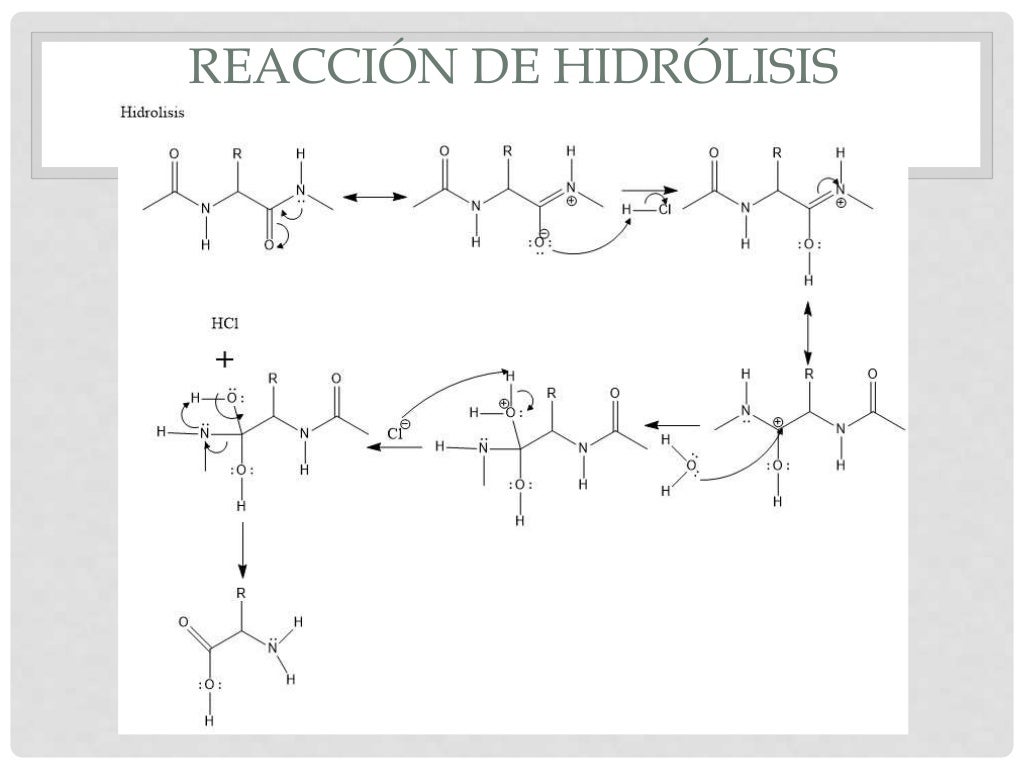
Práctica 6 Hidrólisis de una proteína y ensayos para proteínas y am…
Reaksi hidrolisis garam Na 2 CO 3 adalah . CO 3 2 − + 2 H 2 O ⇌ H 2 CO 3 + 2 OH −. Garam Na 2 CO 3 merupakan garam basa, karena ketika garam ini dilarutkan maka anion akan bereaksi dengan air menghasilkan ion OH-. Pernyataan tersebut dapat dibuktikan melalui reaksi-reaksi yang terjadi sebagai berikut: