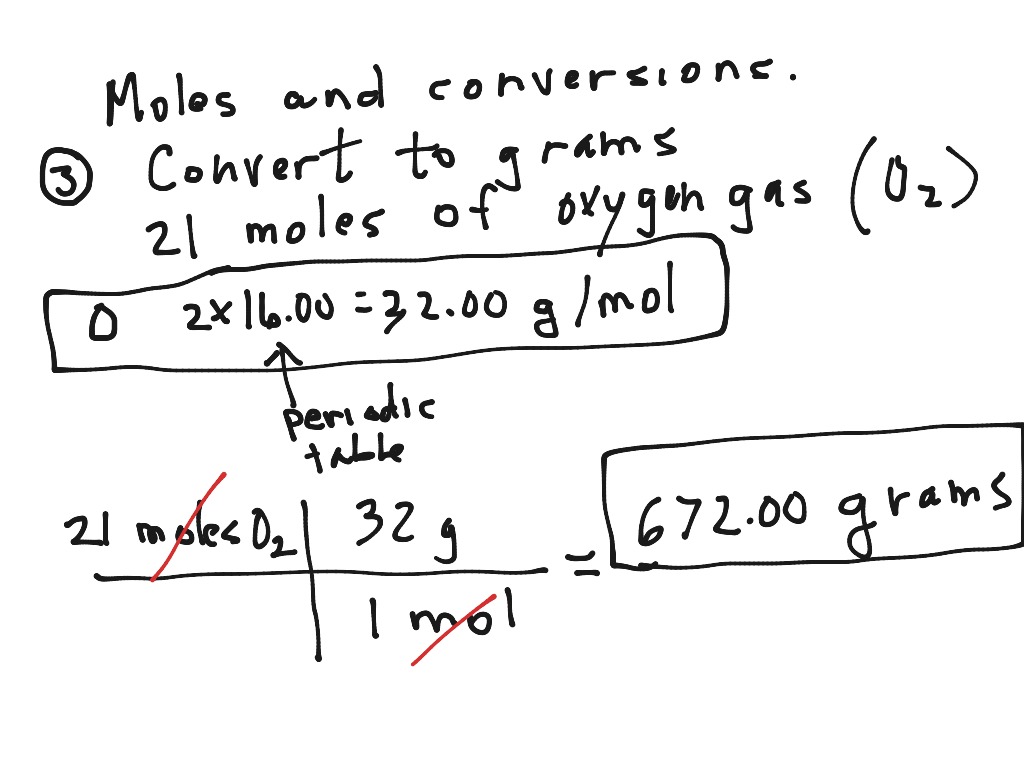
Convert moles of a gas to grams Science, Chemistry, molar mass conversions ShowMe
Group A streptococcal infections are a number of infections with Streptococcus pyogenes, a group A streptococcus (GAS). S. pyogenes is a species of beta-hemolytic Gram-positive bacteria that is responsible for a wide range of infections that are mostly common and fairly mild. If the bacteria enter the bloodstream an infection can become severe and life-threatening, and is called an invasive.

Jika 2 gram gas H_(2) direaksikan dengan 8 gram gas O_...
Graham's law. Graham's law of effusion (also called Graham's law of diffusion) was formulated by Scottish physical chemist Thomas Graham in 1848. [1] Graham found experimentally that the rate of effusion of a gas is inversely proportional to the square root of the molar mass of its particles. [1] This formula is stated as: Rate 1 is the rate of.

Biochemical test reaction interpretation of Gram negative bacterium TSI
This online calculator converts grams to liters and liters to grams given a gas formula. It uses molar volume of a gas at STP (standard temperature and pressure) This calculator finishes the topic started in Convert moles to liters and liters to moles calculator. Because the molar volume is the same for all ideal gases and is known, we can.

Kosangas 450 g gas container Buy cheap gas canisters here
Umarex Unisex's C1250 12gram Airgun Cartridge 50 Pack-12 Gram Powerlets Bulbs Suits All Popular Co2 Pellet and BB Guns Crosman, Gamo and Swith Wesson, etc, Silver, 12g.. Laxzo ® 12g CO2 Cartridges Gas Capsule Cylinder 12 Gram Powerlets Suits All CO2 Pistols an CO2 Rifles, Ideal for Quickly Inflating Bicycle Tyres (Pack of 20).

Gas Laws Using the Ideal gas law to solve for grams YouTube
Conversions Between Moles and Gas Volume. Molar volume at STP can be used to convert from moles to gas volume and from gas volume to moles. The equality of 1mol = 22.4L 1 mol = 22.4 L is the basis for the conversion factor. Many metals react with acids to produce hydrogen gas. A certain reaction produces 86.5 L 86.5 L of hydrogen gas at STP.

Menghitung Volum Gas Pada Suhu dan Tekanan Tertentu YouTube
More information from the unit converter. How many grams GaS in 1 mol? The answer is 101.788. We assume you are converting between grams GaS and mole.You can view more details on each measurement unit: molecular weight of GaS or mol This compound is also known as Gallium(II) Sulfide.The SI base unit for amount of substance is the mole. 1 grams GaS is equal to 0.0098243407867332 mole.

Fraksi mol CO dalam campuran 22 gram gas CO2 dan 28 gram gas CO adalah…( Ar C = 12; Ar O = 16
Cold weather screw-in gas cartridge 230 grams extreme temperature. FORCLAZ Cold weather screw-in gas cartridge 230 grams extreme temperature (78) Currently out of stock online. 4.7/5 Based on 850 Reviews collected online and in stores. Camping Gas Bottles.

20 Gram Co2 Cartridge, NonThreaded 2 Pack
Beans and legumes are known for causing gas. Beans contain high amounts of a complex sugar called raffinose, which the body cannot digest. Beans are also fiber-rich, and a high fiber intake can.

Reaksi Antara Gram Gas Hidrogen Dengan Gram Gas My XXX Hot Girl
1-16 of 97 results for "190 gram gas cartridge" Results. Price and other details may vary based on product size and colour. KARAN KING 12 X Butane Gas/Propane Gas 190g Blowtorch, BBQ, Gas Weed Burner EN417 and EN521 Gas Canister Gas Cartridge Gas Cylinders for Camping, Camping Gas Canister for Camping Stove.
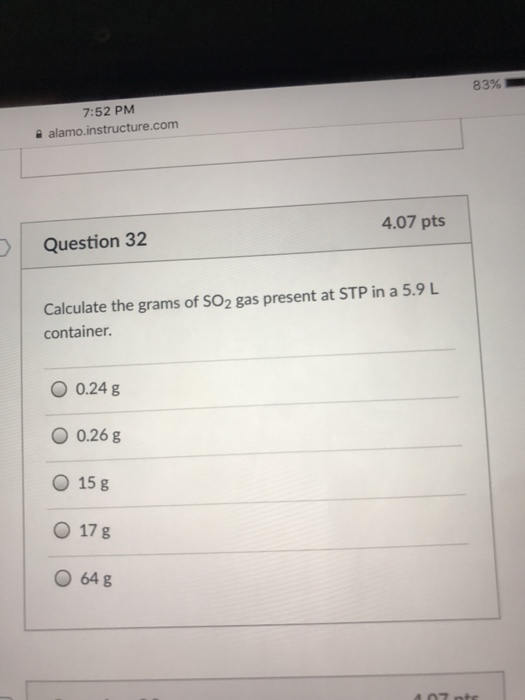
Solved Calculate the grams of SO_2 gas present at STP in a
Volume of the gas (ml, L, dm³, m³); and. Mass (not required for number of moles calculations). Our gas law calculator uses the following equations: The modified ideal gas law formula: Moles = (Pressure × Volume) / (0.0821 × Temperature) If you want to work it out yourself, without the molar mass of gas calculator, be careful with the units.

How to Convert Grams O2 to Moles of O2 to Liters YouTube
Don't forget when buying a Cosiscoop you need a 190-gram gas cartridge. They are available at any reputable gas retailer but for your ease, we have them here too. Burn time is approximately 6 hours although keeping a spare is a good idea so your Cosiscoop can keep your outdoor living area cosy and atmospheric until the wee hours! View the.

Grams to Grams Stoichiometry Step by Step YouTube
How our gas density calculator works. Our gas density calculator employs this formula: \rho = MP/RT ρ = MP /RT to find the density of gas. It takes in the pressure, temperature, and molar mass of gas and calculates the density. Because R R is a constant, we do not require you to enter this value. When you enter these values, the molar mass is.
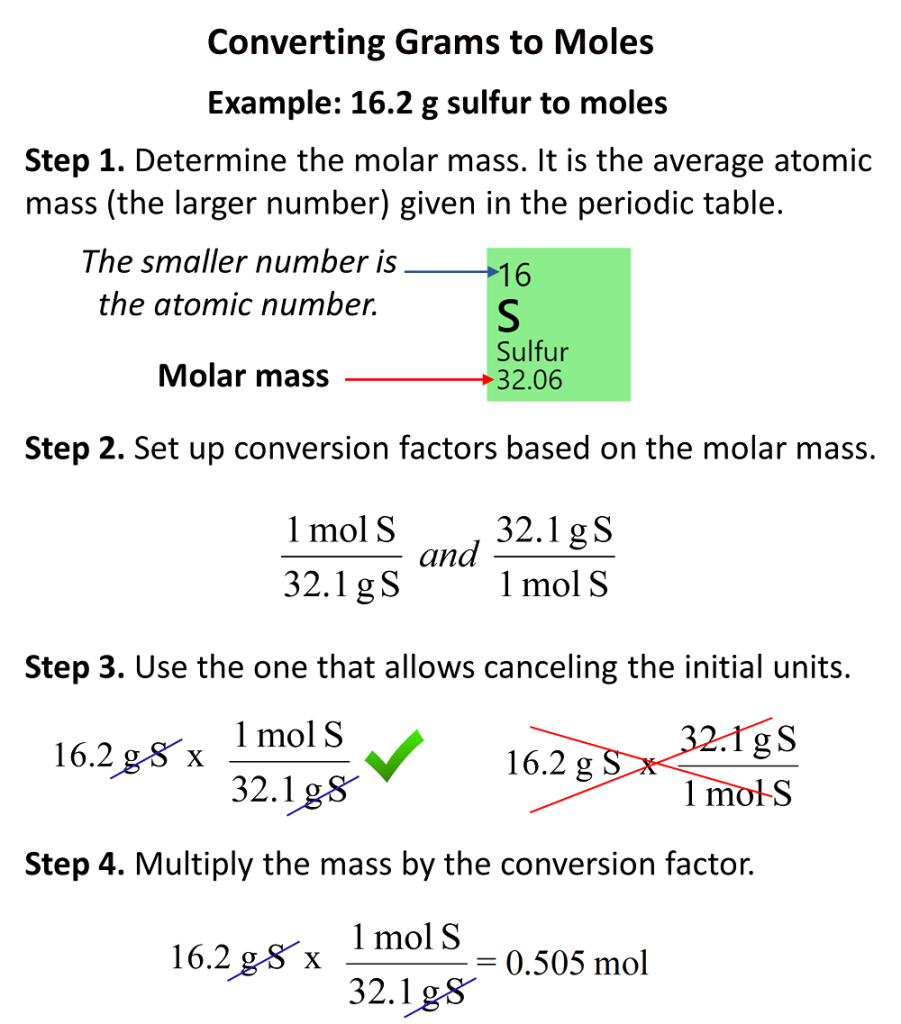
How To Convert Grams To Moles Chemistry Steps
In terms of the average gas bill, the average UK household uses 12,000kWh of gas, which is currently capped at 7p per kWh. This means annually, households will pay an average of £1,923 for gas and electricity (for average use). To find out more about what you can expect to pay, check out our complete guide on appliance running costs and our.
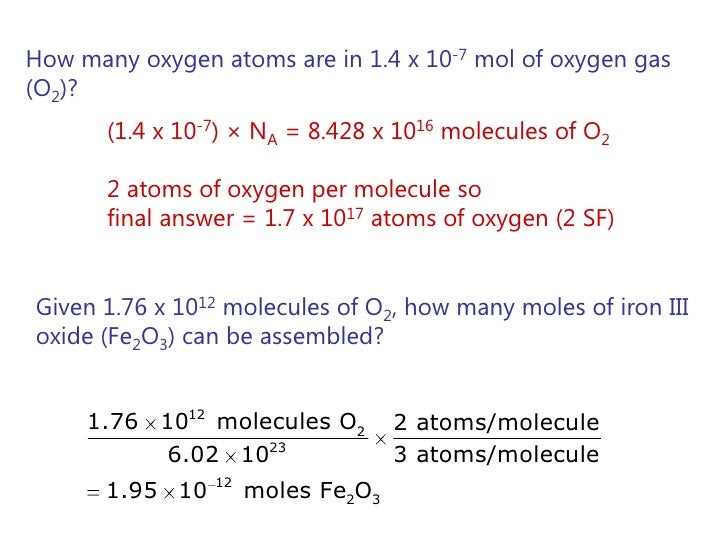
Oxygen Gas Oxygen Gas Grams Per Mole
Soldering Mat 300 x 250mm. ★★★★★ ★★★★★. £5.99. Monument Soldering & Brazing Pad 300 x 300mm. ★★★★★ ★★★★★. £14.98. Choose this 400g propane gas cylinder from Vortex for plumbing and other everyday work. It is a cooler alternative to Vortex Map-X, and is suitable for most brazing or soldering applications.

sebanyak 76 gram campuran gas metana dan etana dib...
To determine this value, we rearrange the ideal gas equation to. n V = P RT (10.5.1) (10.5.1) n V = P R T. Density of a gas is generally expressed in g/L (mass over volume). Multiplication of the left and right sides of Equation 10.5.1 10.5.1 by the molar mass in g/mol ( M M) of the gas gives.

Berapa volume 1 gram gas hidrogen yang diukur pada suhu 25 derajat celcius dan tekanan 1 atom
The molar volume close molar volume The volume occupied by one mole of any gas (24 dm³ or 24000 cm³ at room temperature and pressure). is the volume occupied by one mole of any gas, at room.