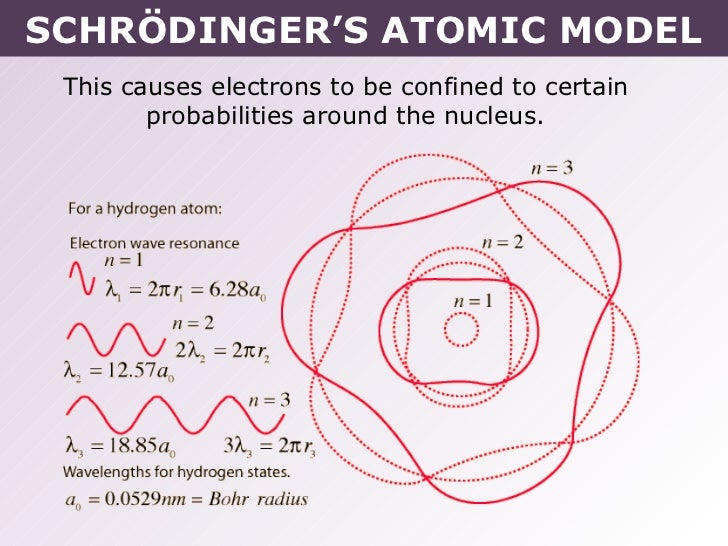
Schrodinger's Model
The theory provided a good description of the spectrum created by the hydrogen atom, but needed to be developed to suit more complicated atoms and molecules. Assuming that matter (e.g., electrons) could be regarded as both particles and waves, in 1926 Erwin Schrödinger formulated a wave equation that accurately calculated the energy levels of electrons in atoms.
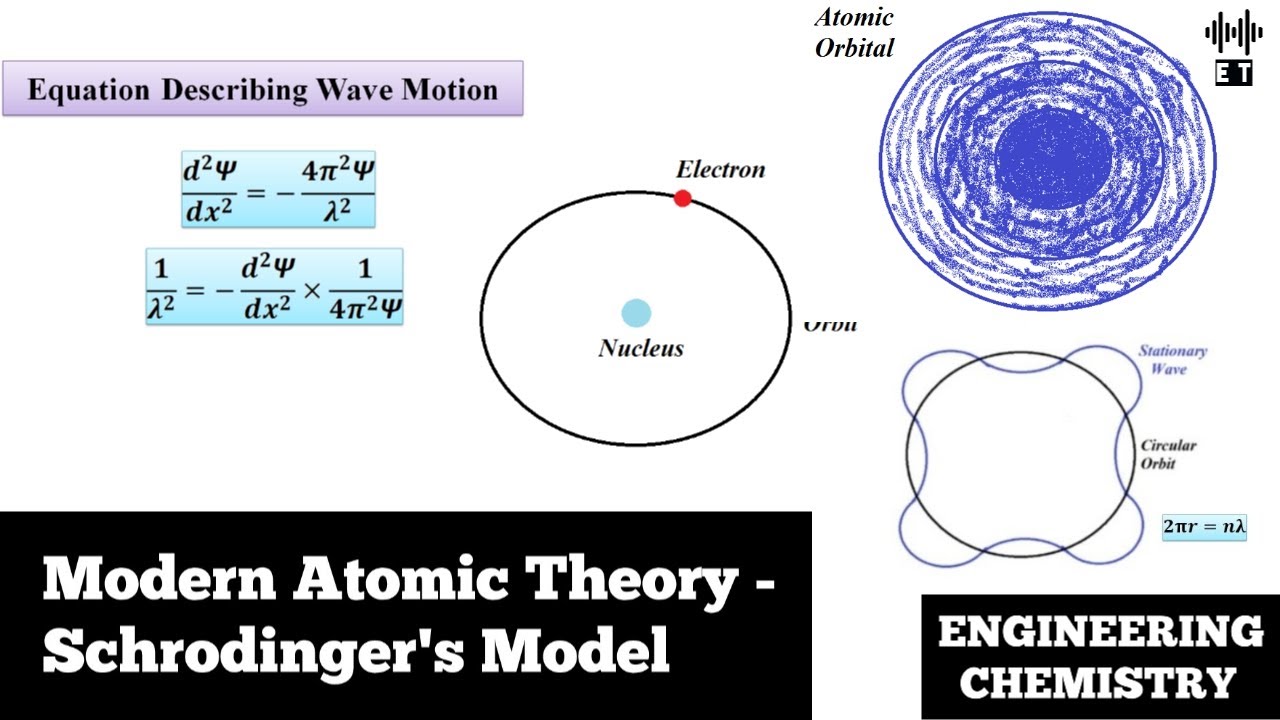
Modern Atomic Theory Schrodinger's Model Basic Concepts Engineering Chemistry YouTube
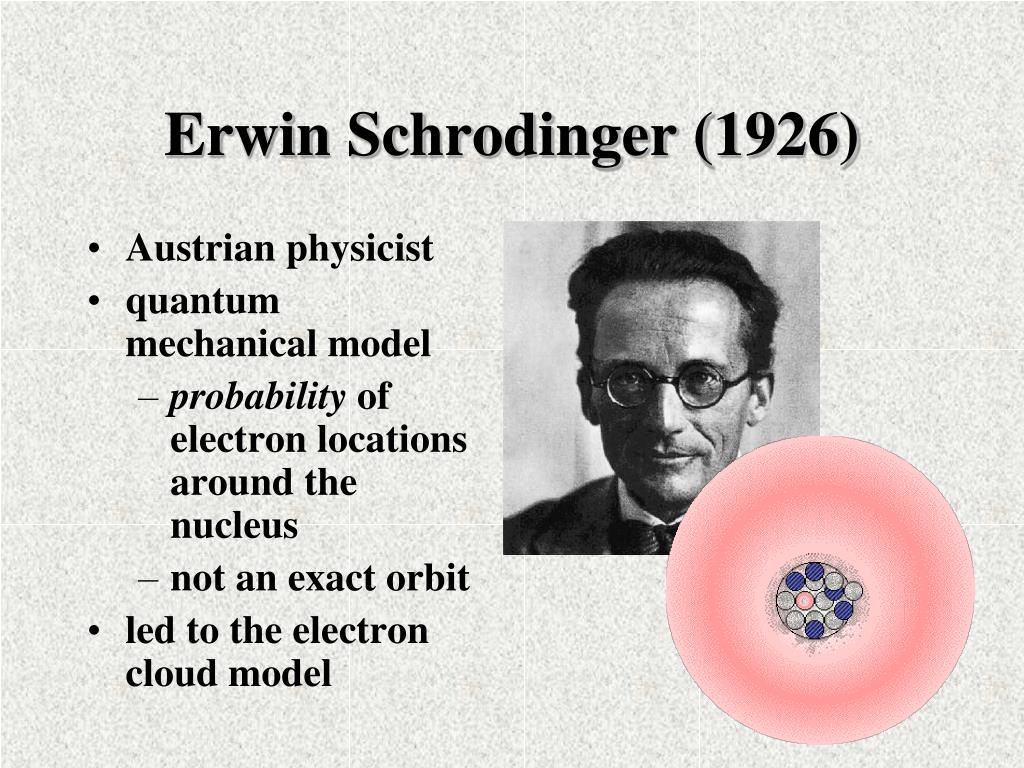
In 1926, an Austrian physicist, Erwin Schrödinger (1887-1961; Nobel Prize in Physics, 1933), developed wave mechanics, a mathematical technique that describes the relationship between the motion of a particle that exhibits wavelike properties and its allowed energies. Schrödinger's wave equation allowed scientists to make predictions about the electronic structure of atoms.

Modelo Atómico de Schrödinger Teoría Atomica
Answer link. The model is known as the electron cloud model or the quantum mechanical model of an atom. The wave equation that he proposed upon being solved gives us a set of three integral numbers known as quantum numbers to specify the wave function of an electron. It was revealed that later a fourth quantum number i.e. the spin quantum.
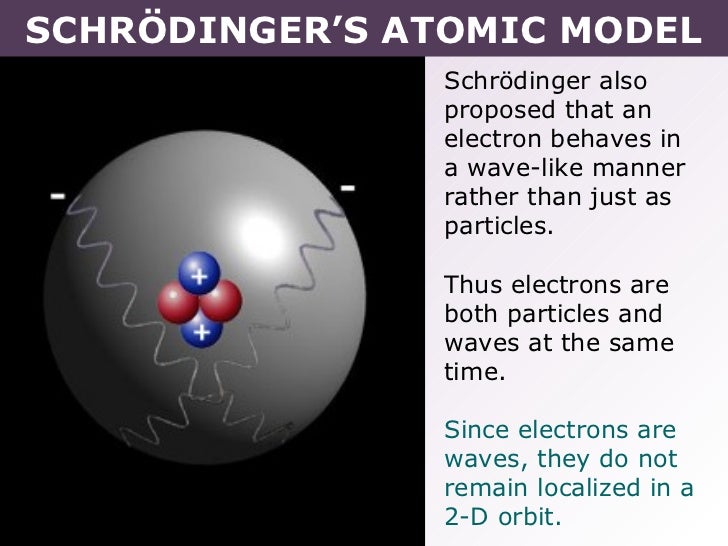
Tang 02 schrödinger’s atomic model
Figure 3.1. 1: Schrödinger's cat: a cat, a flask of poison, and a radioactive source are placed in a sealed box. If an internal monitor detects radioactivity (i.e., a single atom decaying), the flask is shattered, releasing the poison, which kills the cat. The Copenhagen interpretation of quantum mechanics implies that after a while, the cat.

Tang 02 schrödinger’s atomic model
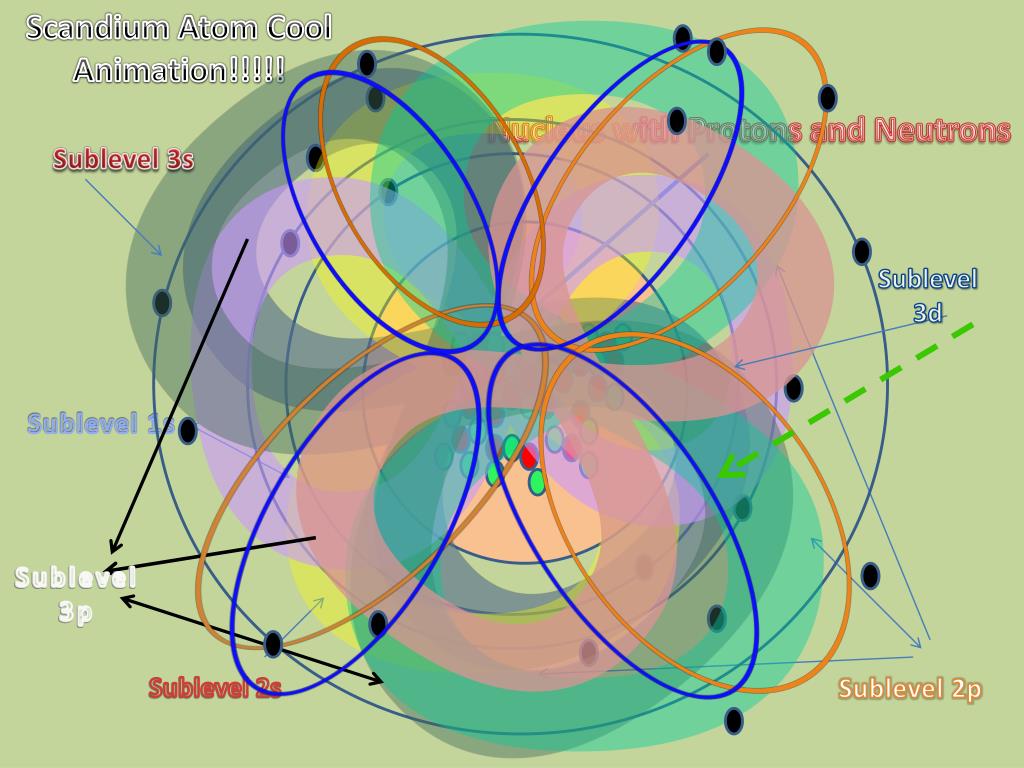
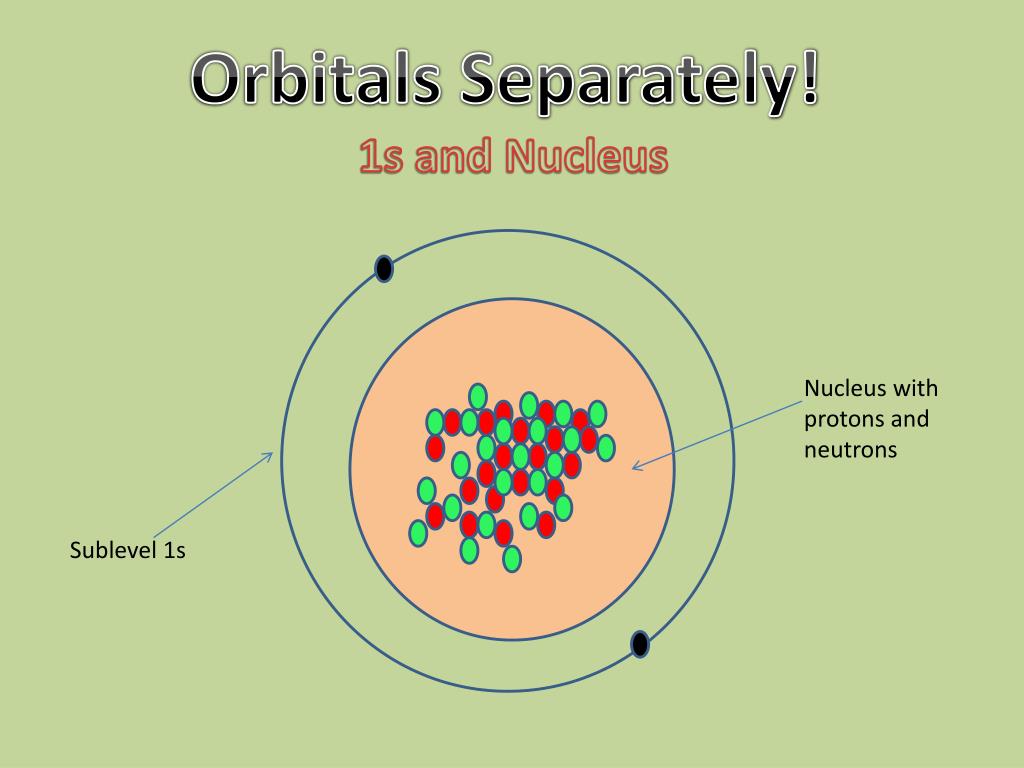
The model of the electron in the nucleus was further developed by the work of Erwin Schrodinger and his development of his wave equation. This equation could be used to solve and give an accurate description of the energy of an electron in an atom due to vibrational modes and the creation of peaks and troughs when these electrons are treated as waves.
Perhatikan Gambar Model Atom Berikut
1 (of 1) The fifth Solvay International Conference on Electrons and Photons, was held in October 1927. Prominent physicists from all the world met to discuss the newly formulated quantum theory. 17 of the 29 participants were or became Nobel Laureates. Back row, left to right: Auguste Piccard, Émile Henriot, Paul Ehrenfest, Édouard Herzen.

Kelebihan Dan Kelemahan Teori Atom Modern bintangutama69.github.io
In 1926 Erwin Schrödinger, an Austrian physicist, took the Bohr atom model one step further. Schrödinger used mathematical equations to describe the likelihood of finding an electron in a certain position. This atomic model is known as the quantum mechanical model of the atom. Unlike the Bohr model, the quantum mechanical model does not.
PPT The Atomic Model Through Time PowerPoint Presentation, free download ID5318415
Erwin Schrödinger (born August 12, 1887, Vienna, Austria—died January 4, 1961, Vienna) Austrian theoretical physicist who contributed to the wave theory of matter and to other fundamentals of quantum mechanics. He shared the 1933 Nobel Prize for Physics with British physicist P.A.M. Dirac.
PPT Erwin Schrödinger’s Model of the Atom Element Representation PowerPoint Presentation ID
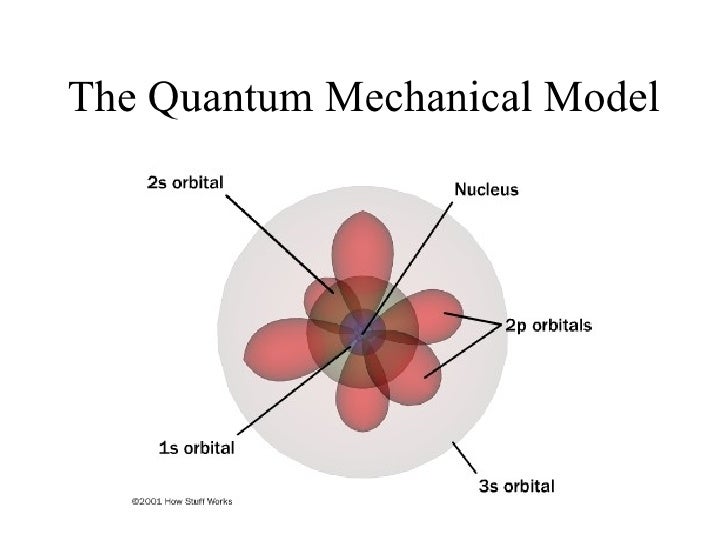
Schrödinger's Contribution to the Current Model of the Atom. Schrödinger used de Broglie's matter wave theory to develop a probabilistic model of the atom. In Schrödinger's model, electrons do not follow sharply defined orbits (like in Bohr's model), but rather are found in orbitals. In addition, Schrödinger's atomic model is based on.

A Century of Quantum Physics Erwin Schrodinger
given by the following equation: λ = h m v. Erwin Schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, H ^ ψ = E ψ. . , can be solved to yield a series of wave function ψ. . , each of which is associated with an electron binding energy, E. .
6. Erwin Schrodinger (18871961) Atomic Theory Timeline
The Erwin Schrödinger model of the atom is composed of the nucleus of the atom which contains protons and neutrons and is surrounded by an electron cloud. This is sometimes called the cloud model.

33.THE ATOMIC STRUCTURE Schrödinger Equation. madoverchemistry
Erwin Rudolf Josef Alexander Schrödinger (UK: / ˈ ʃ r ɜː d ɪ ŋ ə, ˈ ʃ r oʊ d ɪ ŋ ə /, US: / ˈ ʃ r oʊ d ɪ ŋ ər /; German: [ˈɛɐ̯vɪn ˈʃʁøːdɪŋɐ]; 12 August 1887 - 4 January 1961), sometimes written as Schroedinger or Schrodinger, was a Nobel Prize-winning Austrian and naturalized Irish physicist who developed fundamental results in quantum theory.
PPT Erwin Schrödinger’s Model of the Atom Element Representation PowerPoint Presentation ID
A powerful model of the atom was developed by Erwin Schrödinger in 1926. Schrödinger combined the equations for the behavior of waves with the de Broglie equation to generate a mathematical model for the distribution of electrons in an atom. The advantage of this model is that it consists of mathematical equations known as wave functions that.
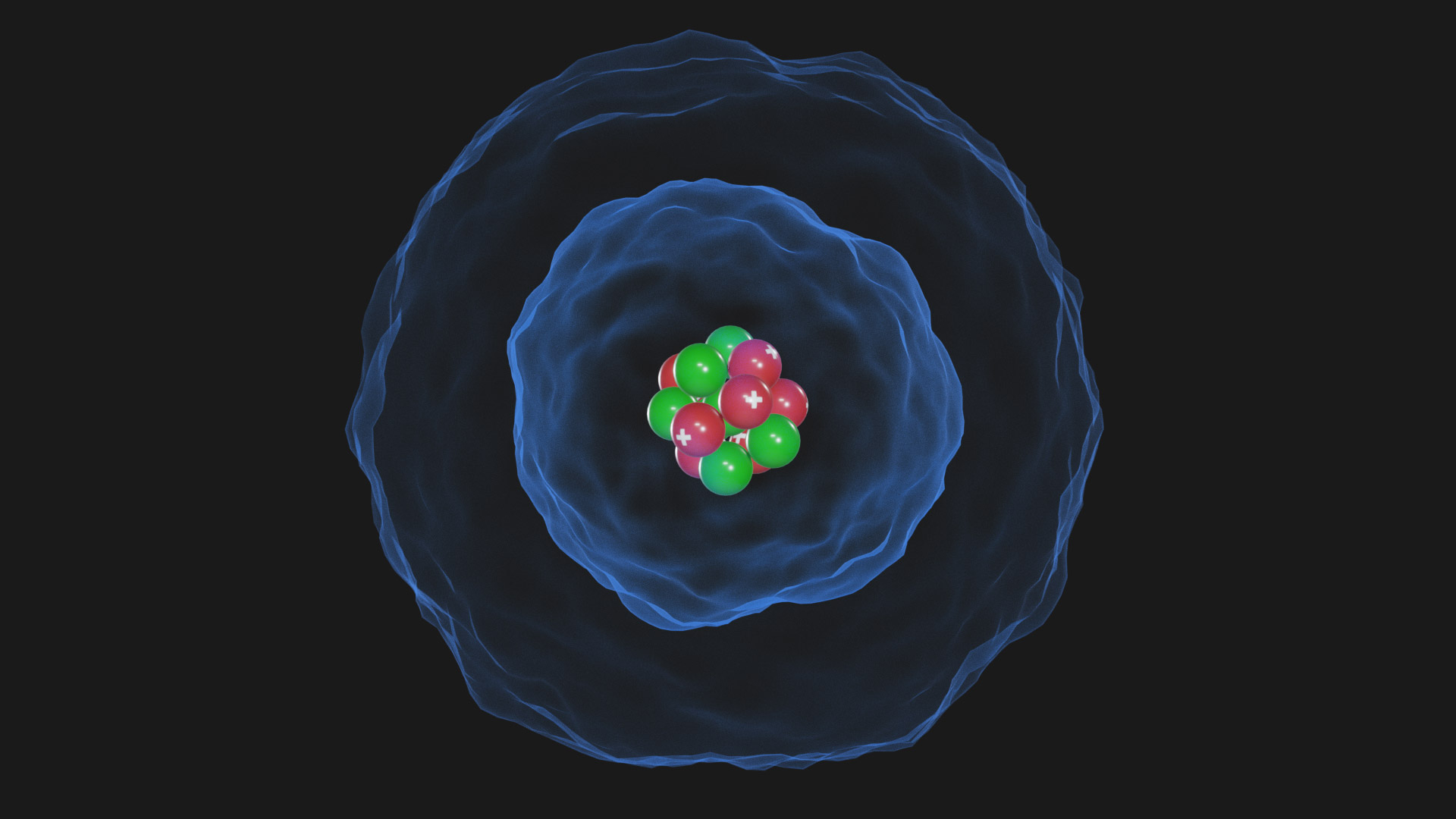
3D Atom Quantum Model Schrodinger model TurboSquid 1770104
📖 Visit our website: http://www.scienceready.com.au 🏅 Become a Patron: https://www.patreon.com/scienceready🎶 Follow our Tiktok https://www.tiktok.com/@hs.
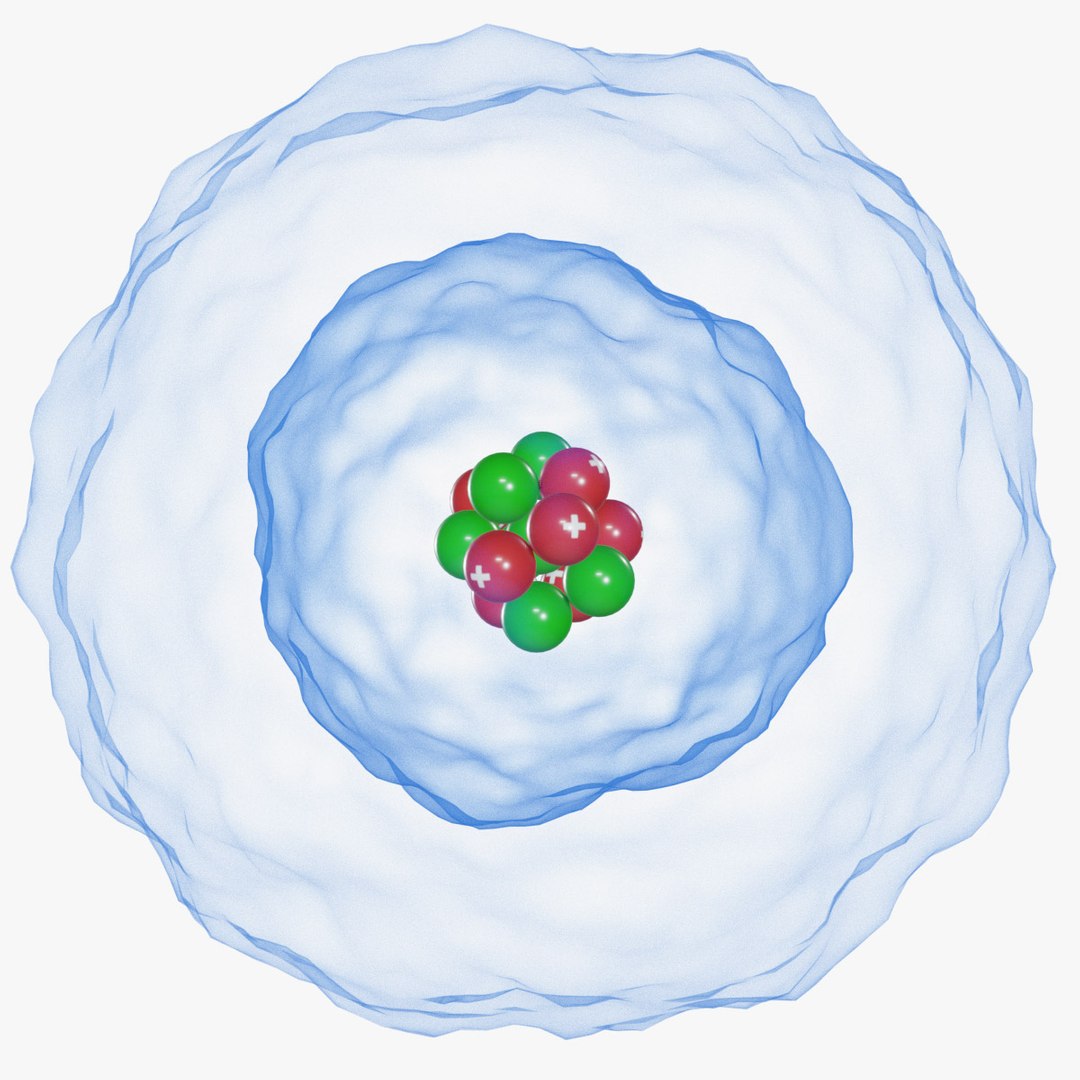
3D Atom Quantum Model Schrodinger model TurboSquid 1770104
QUICK FACTS. Name: Erwin Schrödinger. Birth Year: 1887. Birth date: August 12, 1887. Birth City: Vienna. Birth Country: Austria. Gender: Male. Best Known For: Erwin Schrödinger was a Nobel Prize.

Modelo Atómico de Schrödinger Cursos Online Web
Teori Mekanika Kuantum yang dikembangkan Erwin Schrodinger, Max Planck & ahli lainnya, hingga model atom mekanika kuantum dibahas lengkap di artikel ini. Yuk, belajar bareng!. Model atom mekanika kuantum (sumber gambar: www.sutori.com) Sebelum lanjut, yuk download dulu aplikasi Zenius. Elo bisa dapetin akses ke ribuan materi pelajaran.