
PT phase diagram of Pb. Solid lines represent phase boundaries... Download Scientific Diagram
The Materials Project offers a powerful tool for exploring phase diagrams of various materials, based on computational data and thermodynamic models. You can search for phase diagrams by elements, compounds, or materials ids, and visualize the stability, composition, and energy of different phases. You can also compare your results with experimental data and other sources.
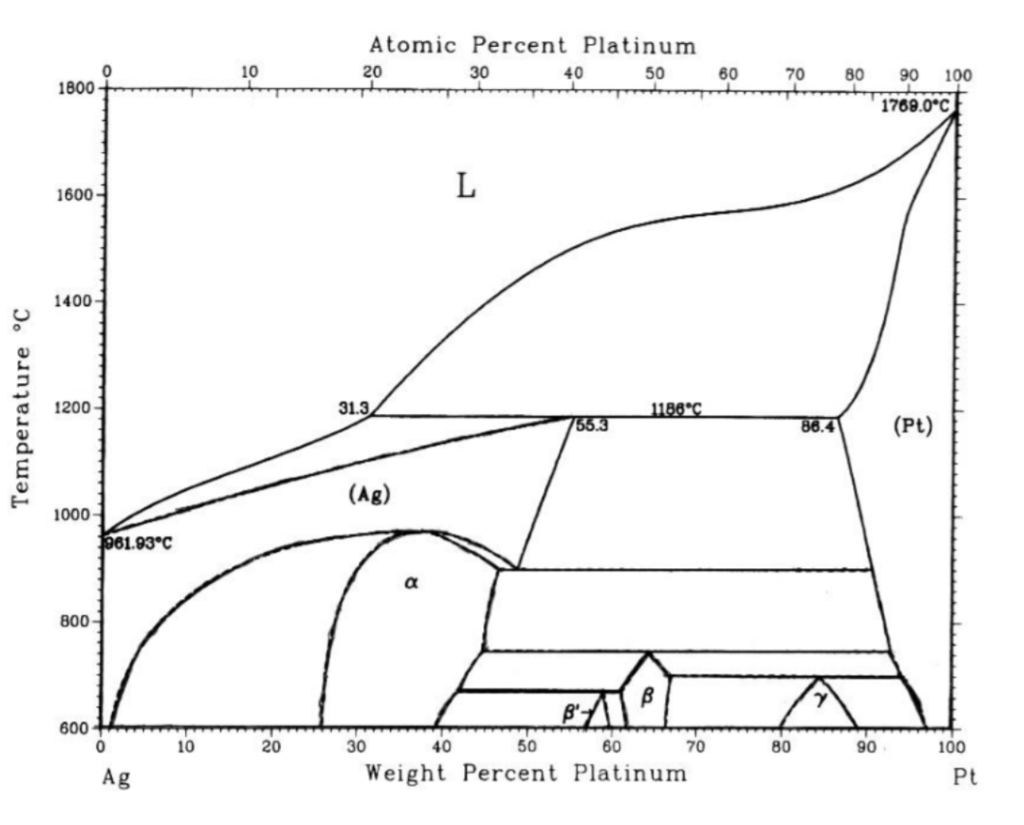
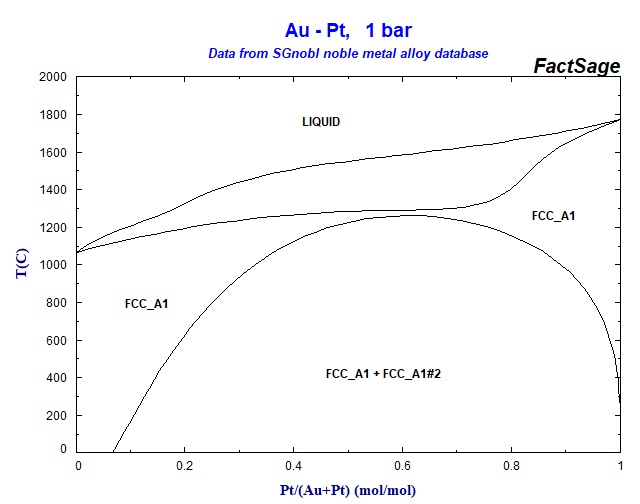
Solved Use the attached AgPt phase diagram to answer A
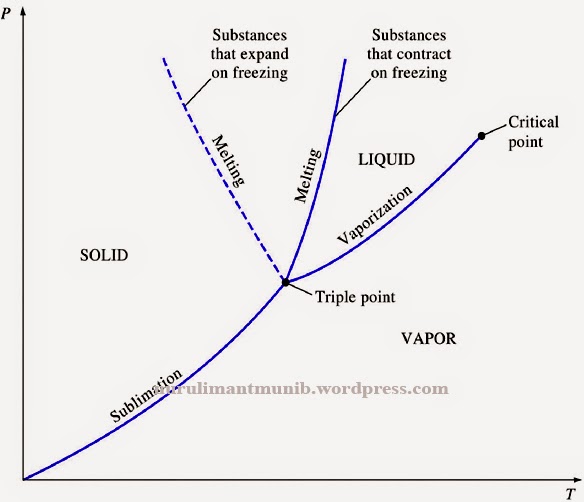
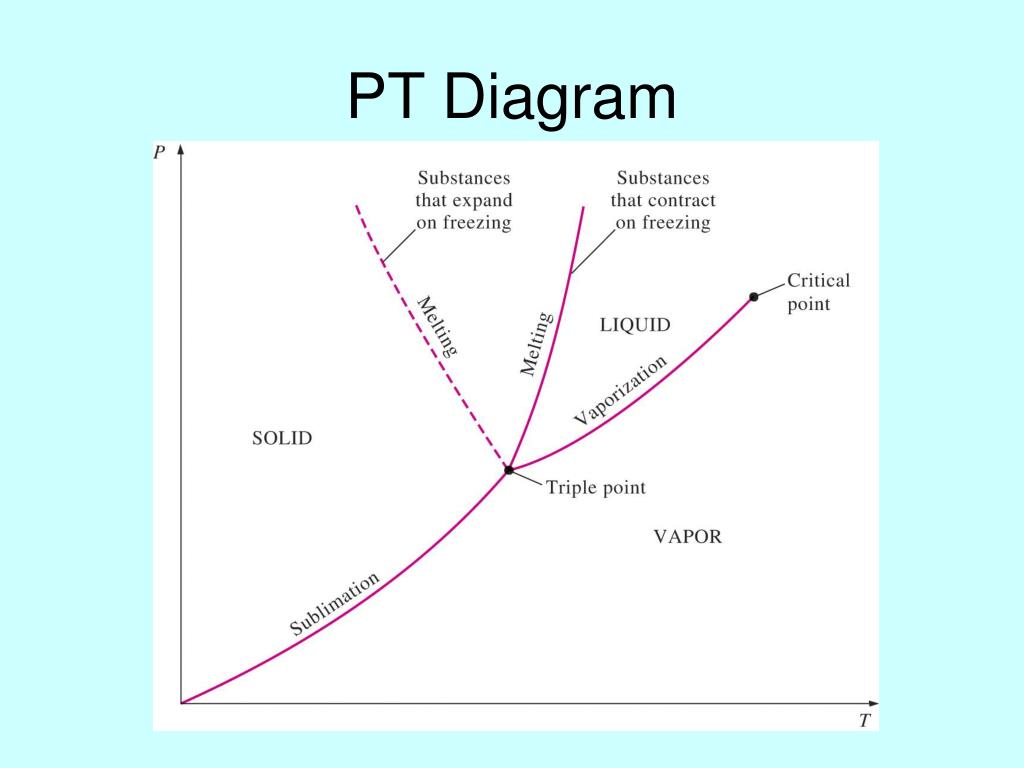
Ch 2, Lesson B, Page 8 - P-T Diagram for a Substance That Expands on Freezing. As I mentioned back on page 6, there are two different types of materials: ones that expand when they freeze and ones that contract when they freeze. By expanding, the solid phase has a larger molar volume than the liquid with which it is in equilibrium.

Phase Changes 1 PT Diagrams YouTube
An important part of using P-T paths or P-T-t paths to understand metamorphic and tectonic processes is to relate the P-T conditions, path shape, and (if age information is available) duration and rate of P-T path segments to the driving forces of metamorphism. P-T path shape by itself does not provide a unique interpretation of tectonic.
Thermodynamics Chapter Summary CHAPTER 3 PROPERTIES OF PURE SUBSTANCES
Phase Diagrams. The plots of pressure versus temperatures provide considerable insight into thermal properties of substances. There are well-defined regions on these graphs that correspond to various phases of matter, so PT PT graphs are called phase diagrams. Figure 13.29 shows the phase diagram for water. Using the graph, if you know the.
PPT Pure Substances PowerPoint Presentation, free download ID485693
The Phase Diagram of Carbon Dioxide. In contrast to the phase diagram of water, the phase diagram of CO 2 (Figure \ (\PageIndex {3}\)) has a more typical melting curve, sloping up and to the right. The triple point is −56.6°C and 5.11 atm, which means that liquid CO 2 cannot exist at pressures lower than 5.11 atm.

1 PT diagram of a pure substance, and thermodynamic states of... Download Scientific Diagram
Phase Diagrams. The phase of a given substance depends on the pressure and temperature. Thus, plots of pressure versus temperature showing the phase in each region provide considerable insight into thermal properties of substances. Such a pT graph is called a phase diagram. Figure \(\PageIndex{1}\) shows the phase diagram for water.
Collection of Phase Diagrams
Fluid Phase Behavior: Pressure-Temperature Diagram (P-T Diagram) Figure 5‑2 shows a P-T diagram for a pure component. The line connecting the triple point and critical points is the vapor pressure curve; the extension below the triple point is sublimation point. As this figure shows in pure materials, by decreasing the pressure at a fixed.

Phaseboundary curves of water in a pT diagram. Download Scientific Diagram
Pressure Temperature (P-T) Diagram. A P-T diagram is the most common way to show the phases of a substance. Figure 9 is the P-T diagram for pure water. A P-T diagram can be constructed for any puresubstance. The line that separates the solid and vapor phases is called the sublimation line.The line that separates the solid and liquid phases is.

PT Diagram for different reservoir fluids Download Scientific Diagram
The PT Projection. On this page we will examine aspects of the pressure-temperature (or PT) diagram. The PT diagram for a pure substance is shown to the right. As before, click on the hyperlink of a particular aspect to see how it appears on this figure. First, we will talk about the vapor pressure curve which is shown in red.
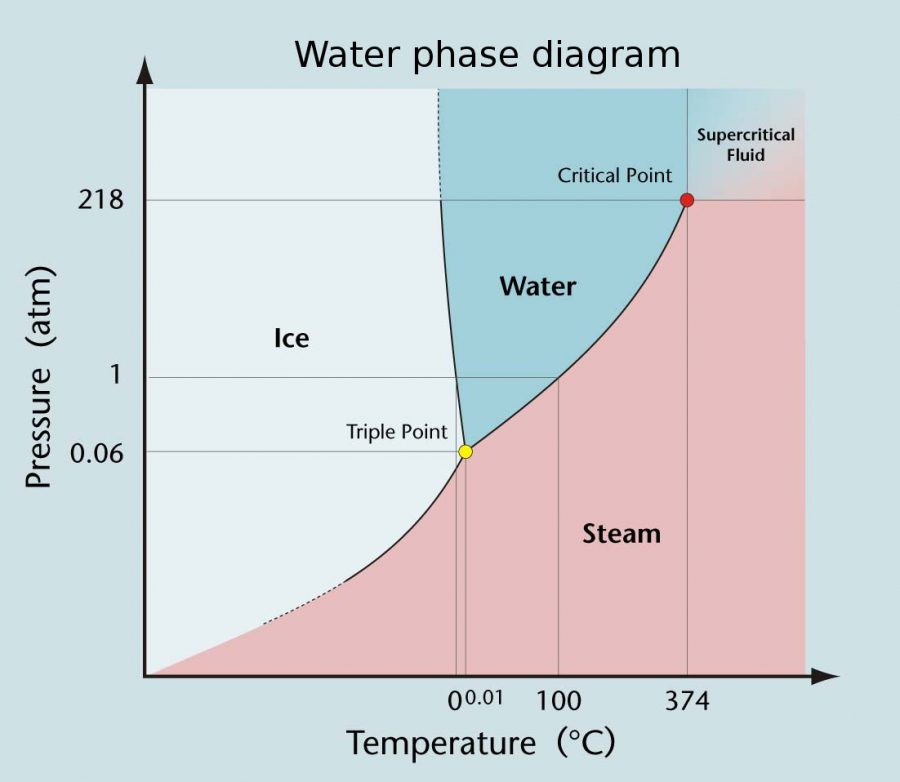
Triple Point of Water The Temperature Where All Three Phases Coexist NA Eye
Phase behavior for water is represented on a log pressure versus temperature diagram. Use the first drop-down menu to select for either an isothermal or isobaric process. Select the particular phase transition (sublimation, melting, vaporization, triple point) from the second drop-down menu. For isobaric transitions, you can add heat with the.

Diagram P, T atau Diagram Fase Air dan Larutan
Pressure Temperature (P-T) Diagram and Equation. A P-T Diagram for Water type of property diagram. Figure 10 is the P- T diagram for pure water. A P-T diagram can be constructed for any pure substance. A P-T diagram is different from a P-T diagram in one particularly important way. There are regions on a P-n diagram in which two phases exist.

Phase Change Diagram of Water — Overview & Importance Expii
Simplified temperature/pressure phase change diagram for water. The pressure on a pressure-temperature diagram (such as the water phase diagram shown above) is the partial pressure of the substance in question.. A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which.
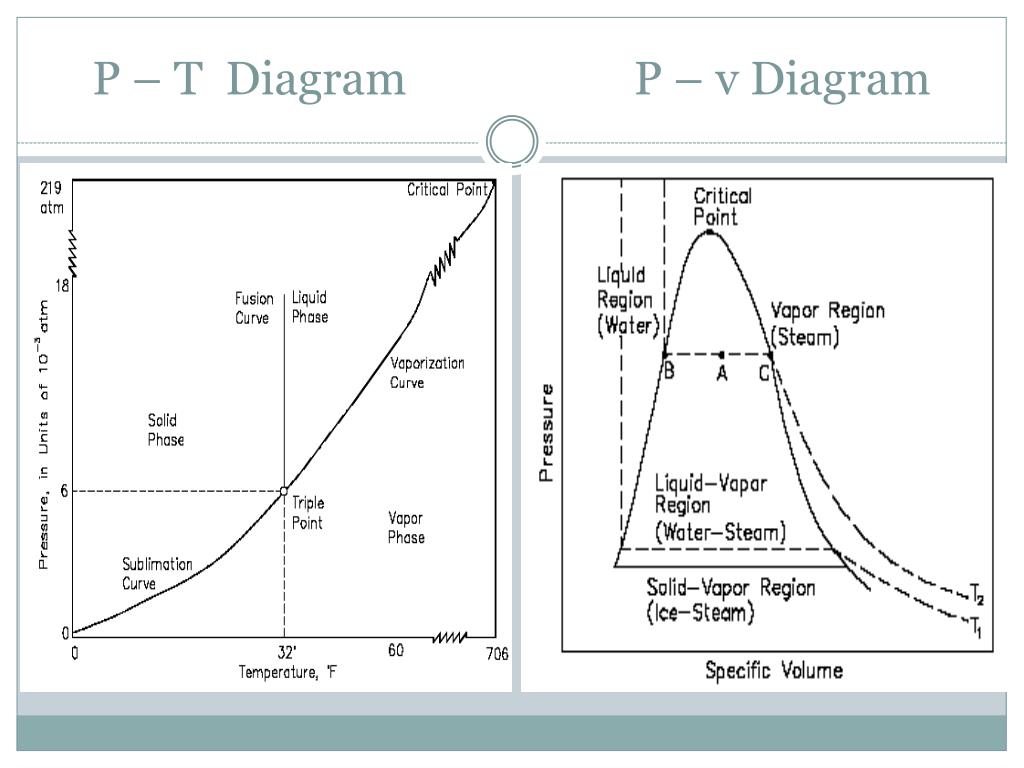
Diagram Pt Kimia Materi Soal
DiagramGPT uses a deep learning model fine-tuned on millions of text-diagram pairs to understand text descriptions and generate corresponding diagrams. It supports a wide range of diagram types including flow charts, sequence diagrams, user journey maps, architecture diagrams, and more. The interface is simple - users just type or paste a text.
Collection of Phase Diagrams
Organized by textbook: https://learncheme.com/ Explains the pressure-temperature and pressure-volume phase diagrams for single component. Made by faculty at.
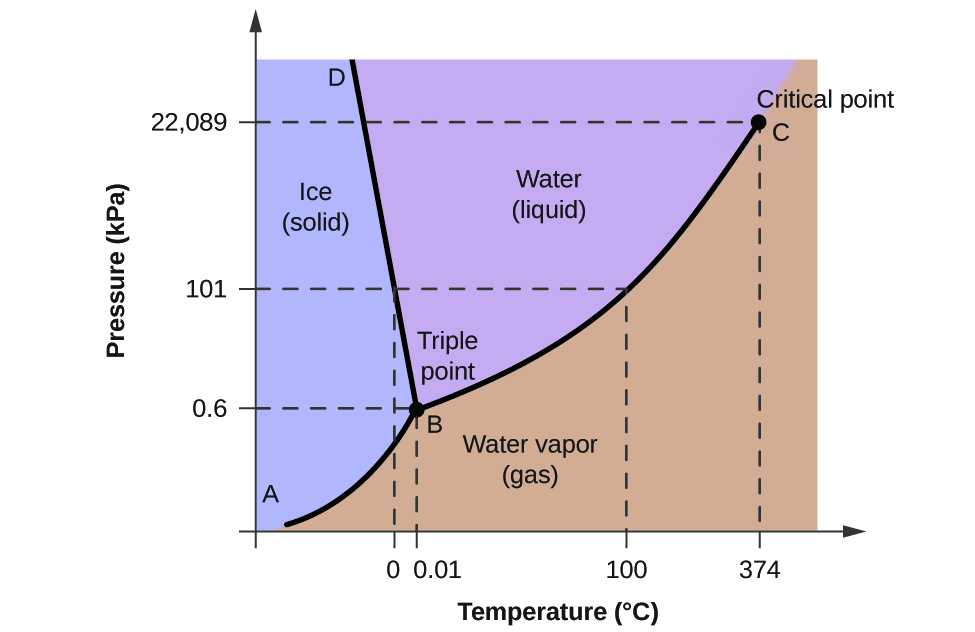
10.4 Phase Diagrams Chemistry LibreTexts
Untuk lebih memahami materi sifat koligatif larutan khususnya penurunan tekanan uap, kenaikan titik didih, dan penurunan titik beku. Perhatikan contoh soal sifat koligatif larutan diagram P-T dan pembahasannya berikut ini. 4 Contoh Soal Sifat Koligatif Larutan Diagram P-T (Fasa) dan Pembahasannya Soal No. 1 Perhatikan diagram P-T berikut! Garis beku pelarut ditunjukkan oleh….

What is the phase diagram of water? Socratic
12.3: PT Phase Diagrams. Let's now discuss the pressure-temperature diagram of a typical substance, as reported in Figure 12.3.1 12.3. 1. Each of the lines reported in the diagram represents an equilibrium between two phases, and therefore it represents a condition that reduces the number of degrees of freedom to one.