
Garam CH3COONH4 mengalami hidrolisis total. Reaksi hidrol...
CH3COONH4 ( Kimia SMA XI, Unggul Sudarmo, Erlangga, 2013, h. 251 ) Jawaban : C Pembahasan : Garam yang berasal dari asam kuat dan basa lemah yaitu contohnya NH4Cl jika dilarutkan dalam air akan menghasilkan kation yang berasal dari basa lemah, kation tersebut bereaksi dengan air dan menghasilkan ion H+ yang menyebabkan larutan bersifat asam.

Explain the structure of CH3COONH4 (Ammonium Acetate) Chemistry Acids Bases and Salts
Garam yang bersifat netral, memiliki pH = 7, berasal dari asam kuat dan basa kuat. Contoh: NaCl (natrium klorida), KI (kalium iodida), dan KNO 3 (kalium nitrat). Pengertian Larutan Garam, Sifat, Ciri, Jenis dan Contohnya : Adalah larutan yang didapat dari hasil reaksi asam dan basa. Garam merupakan suatu senyawa.
8. Which of the following increasing order of pH of 0.1M solutions of the compound A HCOONH4 B
Steps to Find the Molar Mass of CH COONH. There are 4 easy steps to finding the molar mass of CH3COONH4 based on its chemical formula. 1. Count The Number of Each Atom. The first step to finding the molar mass is to count the number of each atom present in a single molecule using the chemical formula, CH3COONH4: Atoms.
.jpg)
Hóa chất Ammonium Acetate CH3COONH4 Xilong thí nghiệm
AA. Ana A. 07 Februari 2022 16:36. Diketahui beberapa larutan garam berikut ini: (1) CH3COONa (2) NH4CN (3) NaCl (4) CH3COONH4 (5) NH4Cl Garam di atas yang inengalarni hidrolisis sebagian dan bersifat basa adalah . . .. A.

записати рівняння гідролізу солей Na2CO3 CH3COONH4 AgNO3 Школьные
Apabila nilai Ka lebih besar dibandingkan Kb maka larutan akan bersifat asam. Sebaliknya, jika nilai Kb lebih besar dibandingkan Ka, maka bersifat basa. Lalu, jika nilai Ka dan Kb sama maka sifat larutannya adalah netral. Salah satu contohnya adalah ammonium asetat (CH3COONH4) terbentuk dari basa lemah dan asam lemah.
.jpg)
Ammonium Acetate CH3COONH4
Ammonium acetate, also known as spirit of Mindererus in aqueous solution, is a chemical compound with the formula NH 4 CH 3 CO 2. It is a white, hygroscopic solid and can be derived from the reaction of ammonia and acetic acid. It is available commercially. [5]

Napisz reakcje hydrolizy CH3COONa i CH3COONH4. Brainly.pl
Penentuan pH larutan bergantung pada tetapan kesetimbangan baik Ka maupun Kb dengan memperhatikan syarat berikut. 1. Jika Ka > Kb maka larutan bersifat asam. 2. Jika Kb > Ka maka larutan bersifat basa. 3. Jika Ka = Kb maka larutan bersifat netral. Penentuan pH larutan CH₃COONH₄ sebagai berikut.
.jpg)
Hitunglah ph CH3COONH4 0,1 M (Ka CH3COOH = 1× 105 dan Kb NH3 = 1× 105) Id Sejarah Kita
Sifat garam KCl tergantung kepada jumlah partikel asam kuat dan basa kuatnya, jika jumlah partikelnya sama maka garam bersifat netral. 5. Garam CH3COONH4 CH3COONH4 >>> CH3COO^- + NH4^+ Anion CH3COO- berasal dari asam lemah yaitu CH3COOH, mengalami hidrolisis CH3COO^- + H2O >>> CH3COOH + OH^- Kation NH4^+ berasal dari basa lemah yaitu NH4OH/NH3.

Garam CH3COONH4 mengalami hidrolisis total.Reaksi hidroli...
CH3COONH4 molecular weight. Molar mass of CH3COONH4 = 77.08248 g/mol. This compound is also known as Ammonium Acetate. Convert grams CH3COONH4 to moles. or. moles CH3COONH4 to grams. Molecular weight calculation: 12.0107 + 1.00794*3 + 12.0107 + 15.9994 + 15.9994 + 14.0067 + 1.00794*4

WHICH OF THE FOLLOWING INCREASING ORDER OF PH OF .1 M SOLUTION OF THE COMPOUND AHCOONH4,B
Asam asetat murni sangat mudah terbakar, apalagi jika suhunya mencapai 39 °C. ADVERTISEMENT. CH3COOH mampu bereaksi dengan air dan menghasilkan ion etanoat dan ion hidroksonium. Namun, perlu digarisbawahi bahwa hanya sekitar 1% saja yang mampu bereaksi. Sedangkan sisanya tetap menjadi CH3COOH karena sifatnya yang reversibel.

Купить Ацетат аммония (CH3COONH4) от компании «UkrChemGroup» 864014509
CH3COOH + NH3 = CH3COONH4 + H2O is a Double Displacement (Metathesis) reaction where one mole of Acetic Acid [CH 3 COOH] and one mole of Ammonia [NH 3] react to form one mole of Ammonium Acetate [CH 3 COONH 4] and zero moles of Water [H 2 O] Show Chemical Structure Image. Reaction Type.

Các tính chất của ch3cooh là chất điện li mạnh hay yếu được giải đáp chi tiết
Since there is an equal number of each element in the reactants and products of CH3COONH4 + H2O = CH3COONH3 + H3O, the equation is balanced. Balance CH3COONH4 + H2O = CH3COONH3 + H3O Using Inspection The law of conservation of mass states that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a.

Hóa chất Ammonium AcetateCH3COONH4 CÔNG TY TNHH HOA VIỆT CHEMGROUP
Ammonium acetate can be prepared by reacting acetic acid with ammonia, ammonium bicarbonate or carbonate. Drying the solution by boiling the excess water is difficult, as ammonium acetate will decompose if the temperature is uneven. Heating the solution on a water bath at below 90 °C is an option, though a bit of ammonium acetate will.

Tính chất và ứng dụng của ch3coonh4 là chất điện li mạnh hay yếu?
Phát biểu nào sau đây là chính xác về CH3COONH4? A. Là muối hữu cơ, có tên là amoni axetat. B. Là hợp chất tạp chức có tên là axetata amino. C. Là muối của axit axetic với amoniac, có tên là axetat amino. D. Là hợp chất tạp chức có tên là amoni axetat.
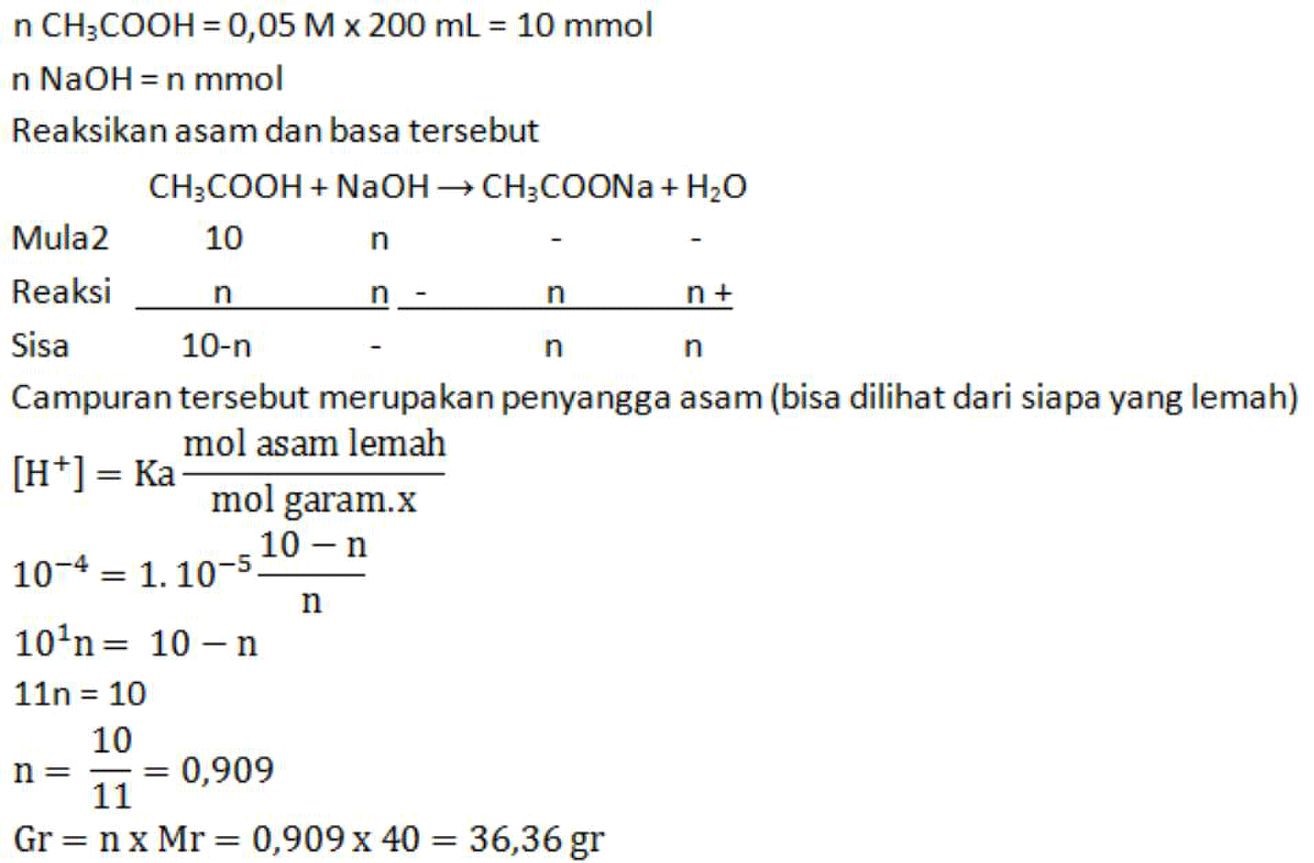
Berapa gram NaOH (Mr = 40) harus dimasukkan ke dalam 200 mL larutan CH3COOH 0,05 M agar didapat
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. Since there is an equal number of each element in the reactants and products of CH3COONH4 + HCl = CH3COOH + NH4Cl, the equation is balanced.

Hitunglah pH larutan dari a. Larutan NaCN 0,1 M (Ka HCN = 4 x 106) b. CH3COONH4 0,1 M (KA
Jawaban: Larutan garam memiliki pH = 7 dan bersifat netral. Derajat keasaman dan kebasaan suatu larutan dinyatakan dengan pH. Larutan CH₃COONH₄ terbentuk dari asam lemah dan basa lemah. Ketentuan sifat garam dari asam lemah dan basa lemah adalah sebagai berikut: 1. Jika Ka = Kb, maka larutan garam bersifat netral.