
Oxalic Acid 1kg / 3kg Factor O
Successive acid dissociation constants are provided for polyprotic weak acids; where there is ambiguity, the specific acidic proton is identified. To find the Kb value for a conjugate weak base, recall that. Ka ×Kb = Kw (E5.1) (E5.1) K a × K b = K w. for a conjugate weak acid, HA, and its conjugate weak base, A -. Compound.
Oxalic acid molecule. Skeletal formula Stock Photo Alamy
The grittiness you feel on your tongue is the oxalic acid reacting with the calcium in your saliva to form oxalates (salts of oxalic acid). 2. It can make the sap irritating to the mouth, skin, or eyes. 3. It ties up calcium in the animal's gut, and cause kidney stones if eaten for extended periods. 4.

Oxalic Acid 5 Solution Stain Remover Trade Chemicals
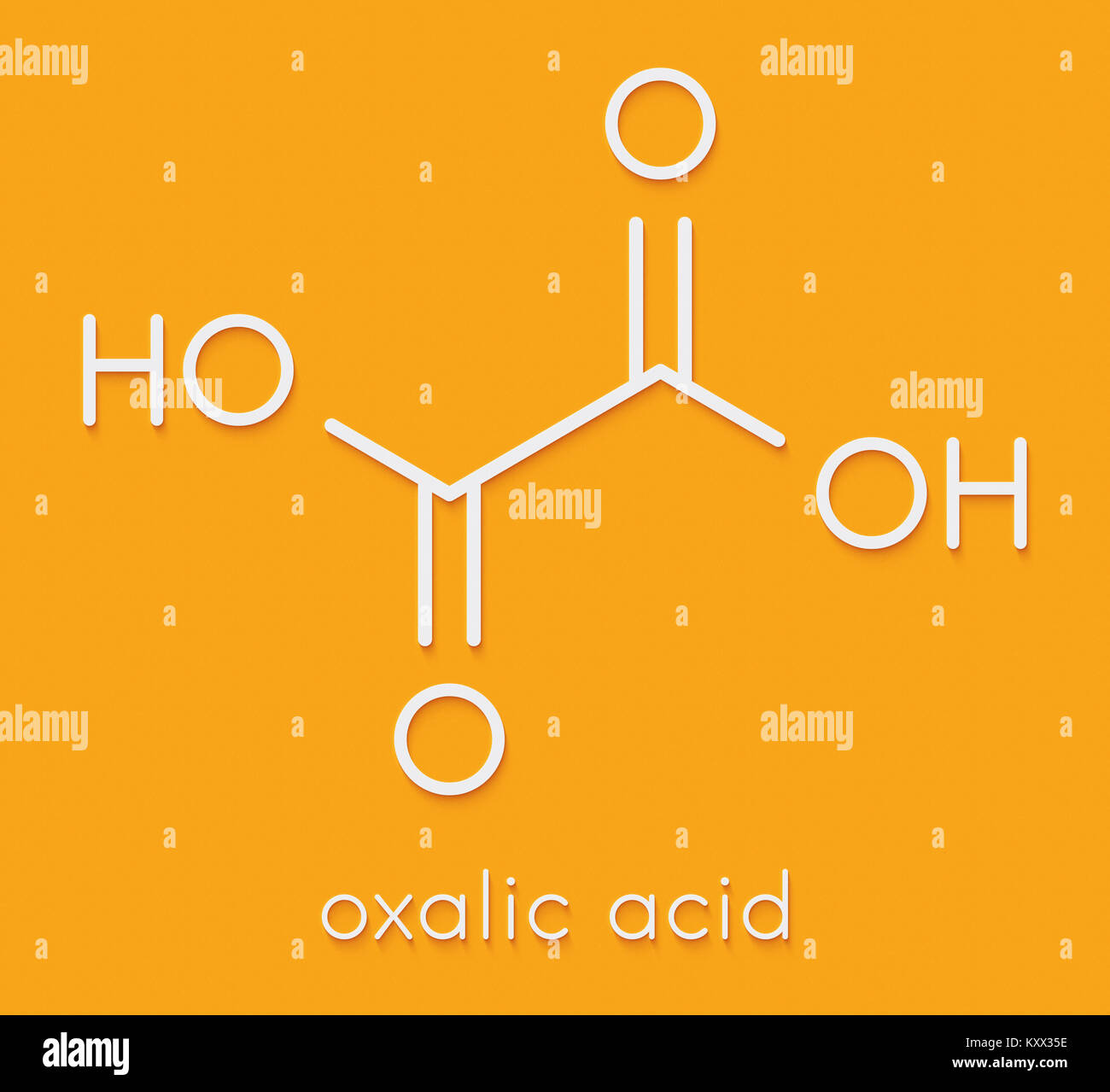
Oxalic acid is an organic acid with the systematic name ethanedioic acid and chemical formula HO−C(=O)−C(=O)−OH, also written as (COOH) 2 or (CO 2 H) 2 or H 2 C 2 O 4.It is the simplest dicarboxylic acid.It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus.

OXALIC ACID 500gm Amazon.in Industrial & Scientific
Oxalate can reduce mineral absorption. One of the main health concerns about oxalate is that it can bind to minerals in the gut and prevent the body from absorbing them. For example, spinach is.

Oxalic Acid Rust Remover Ecochem Limited
The Science Behind Oxalic Acid's Cleaning Power. Oxalic acid acts as a reducing agent, effectively breaking down the chemical bonds that hold rust and stains onto metal surfaces. When oxalic acid comes into contact with rust, it undergoes a chemical reaction, converting the rust into a soluble compound that can be easily rinsed away.

Cleaning cans with oxalic acid YouTube
To use a 12-volt passive vaporizer, the beekeeper measures oxalic acid crystals (from 1-3 grams) into the cooled vaporizer, shoves the device into the entrance, seals the hive entrance up with foam or rags, then energizes the vaporizer with a power cord from the truck battery. The device quickly heats and vaporizes the OA.

Oxalic Acid Hive World natural varroa treatment
Figure 8.9.1 Redox reaction of potassium permanganate and oxalic acid Suppose you stirred a 10.0 g sample of canned rhubarb with enough dilute H 2 SO 4 (aq) to obtain 127 mL of colorless solution. Because the added permanganate is rapidly consumed, adding small volumes of a 0.0247 M KMnO 4 solution, which has a deep purple color, to the rhubarb.

Get Oxalic Acid Safety Data Sheet Pictures Best Information and Trends
In this article, we'll be discussing some precautions and methods you should take when handling oxalic acid. 1. If Inhaled: Oxalic acid vapor is dangerous when inhaled. It causes serious burns to the respiratory tract, throat, and nose. If oxalic acid vapor or dust is inhaled it can cause nervousness, mucous membrane ulcers, vomiting, headache.

Oxalic Acid Dribble Method For Mite Treatment YouTube
Oxalic acid is a dicarboxylic acid with a chemical formula C 2 H 2 O 4. It is also known as Ethanedioic acid or Oxiric acid. This organic compound is found in many vegetables and plants. It is the simplest dicarboxylic acid with condensed formula HOOC-COOH and has an acidic strength greater than acetic acid.

Oxalic Acid Solution
One ounce of almonds, or about 22 nuts, contains 122 milligrams of oxalates. 4. Potatoes. A medium baked potato has 97 milligrams of oxalates per serving. Much of this content is in the potato's.

What is Oxalic Acid & How to Buy Oxalic Acid
Oxalic acid is a common organic compound. A range of living organisms — including fungi, bacteria, plants, animals, and humans — produce it. Technically, oxalate occurs when the oxalic acid in.

Oxalic Acid Treatment Protocols LópezUribe Lab
Oxalic acid is applied to lighten the color and soften the tone of wood and leather. Oxalic acid is a gentle stain remover for stone, brick, linoleum, wood, and vinyl surfaces. It is the best rust removal agent for metals. It is the best polishing agent to give the original shine of marbles and stones.

Oxalic acid tablets 1000g121000
3.2 Textile Treatment. Oxalic acid is utilized as a mordant in the printing and dyeing of wool and cotton. It is used as a stripping agent for wool colors to achieve special pattern effects, as an auxiliary in indigo caustic discharge printing, and in vat dyeing as a reducing agent for potassium dichromate.

First Oxalic Acid Treatment YouTube
Few health problems are linked to oxalic acid, although it can cause formation of kidney stones in people with inflammatory bowel disease, primarily because they are unable to regulate the amount they absorb.(Most kidney stones are composed of calcium oxalate.) For this reason susceptible individuals are advised to follow a low-oxalate diet. (If you've had a kidney stone and want to avoid.

Oxalic Acid, Technical Grade, 99.6 Z Chemicals
The pH of 0.1 M oxalic acid is 1.26, and its high acidity causes calcium phosphate (a component of teeth) to dissolve easily; however, it instantly reacts with calcium to form calcium oxalate monohydrate. The solubility of calcium oxalate in water is 0.00067 g/100 mL (at 20 °C) [ 16 ], which is a minimal value.

Oxalic Acid Ecoxall Chemicals
Oxalic acid in model wastewater mixed with P25 TiO 2 and simulated sunlight (AM1.5) was used with continuous ozone bubbling. To quantify oxalic acid, the catalyst was removed, the solution was mixed with the sensor solution, then the absorbance at 505 nm was measured using a standard UV-Vis spectrophotometer. A limitation identified in the.