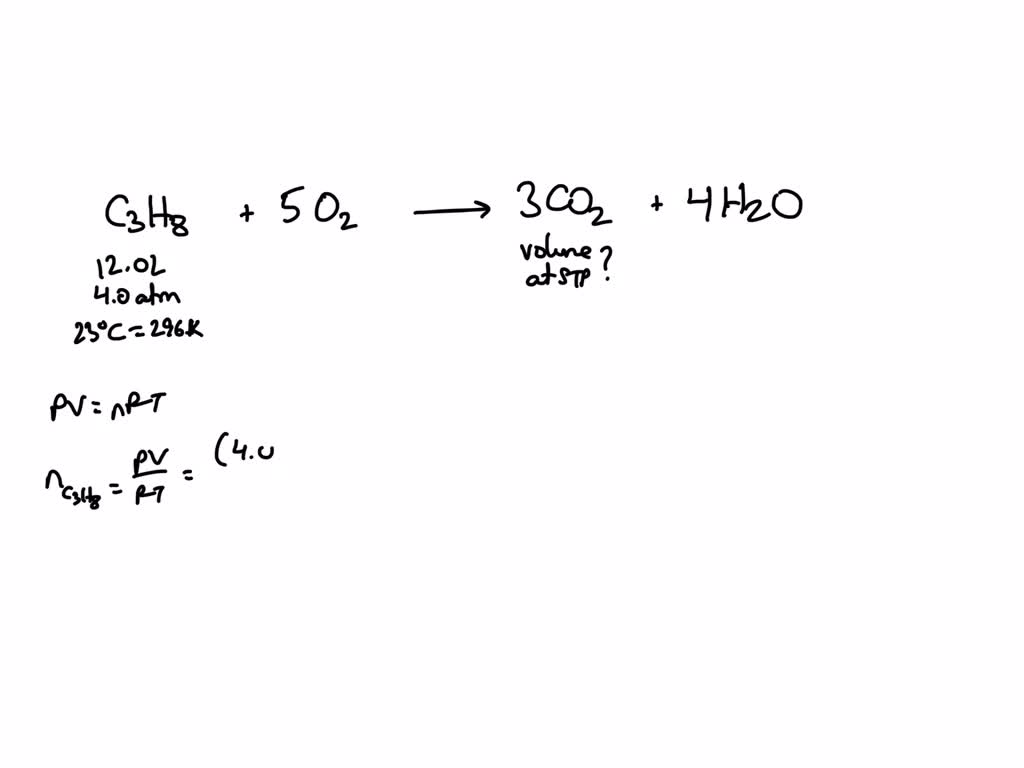
SOLVED Propane gas (C3H8) is used as a fuel in rural areas.How many liters of CO2 are formed at
C3H8 + O2 --> CO2 + H2O. Natural Language; Math Input; Extended Keyboard Examples Upload Random. Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, engineering, mathematics, linguistics, sports, finance, music…

Balance the equation C3H8+O2→CO2+H20??? Brainly.in
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. C3H8 + 5 O2 = 3 CO2 + 4 H2O. Reactants. Products.
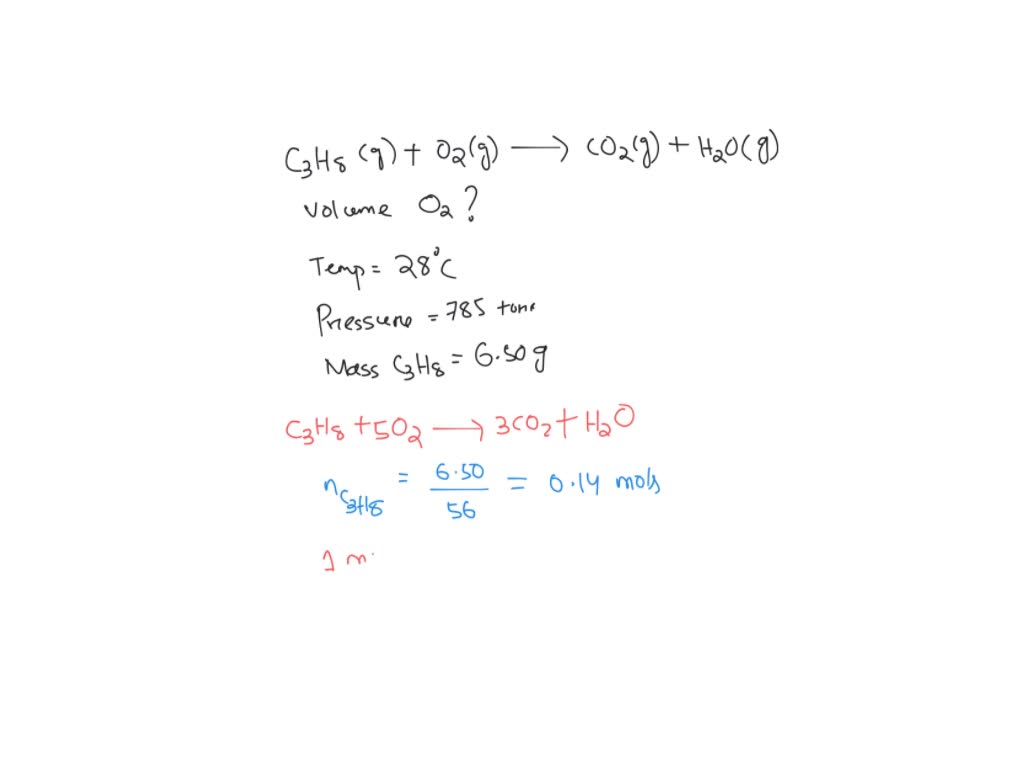
SOLVED Perhatikan persmaan reaksi yang belum setara berikut! C3H8 + O2 > CO2 + H2O
HERMOSA CASA UBICADO EN EXCLUSIO CONDOMINIO LOS TILOS DE BUIN. Ubicada a 30 minutos del Centro de Santiago, excelente conectividad con carreteras, cercano a Metro Tren (Estación Buin Zoo y Linderos), restaurantes, colegios, centros comerciales, supermercados, viñas y mas. La casa cuenta con: - 1 Dormitorio en suite, con gran walking closet. - 3 dormitorios amplios. - 3 baños.

How to Balance C3H8+O2→CO2+H2O YouTube
C3H8 + O2 = CO2 + H2O. The coefficients show the number of particles (atoms or molecules), and the indices show the number of atoms that make up the molecule. New substances are formed as a result of the rearrangement of the original atoms. As a result of a chemical reaction, atoms of chemical elements do not disappear anywhere and new ones do.

How to balance C3H8 + O2 → CO2 + H2O YouTube
Word Equation. Isopropyl Alcohol + Dioxygen = Carbon Dioxide + Water. C3H8O + O2 = CO2 + H2O is a Combustion reaction where two moles of Isopropyl Alcohol [C 3 H 8 O] and nine moles of Dioxygen [O 2] react to form six moles of Carbon Dioxide [CO 2] and eight moles of Water [H 2 O]

SOLVED Enthalpy of formation of C3h8, CO2 H2O a is 104, 394,286 kilo joule per mole
1 C 3 H 8 + 1 O 2 = 1 CO 2 + 1 H 2 O. For each element, we check if the number of atoms is balanced on both sides of the equation. C is not balanced: 3 atoms in reagents and 1 atom in products. In order to balance C on both sides we: Multiply coefficient for CO 2 by 3. 1 C 3 H 8 + 1 O 2 = 3 CO 2 + 1 H 2 O.
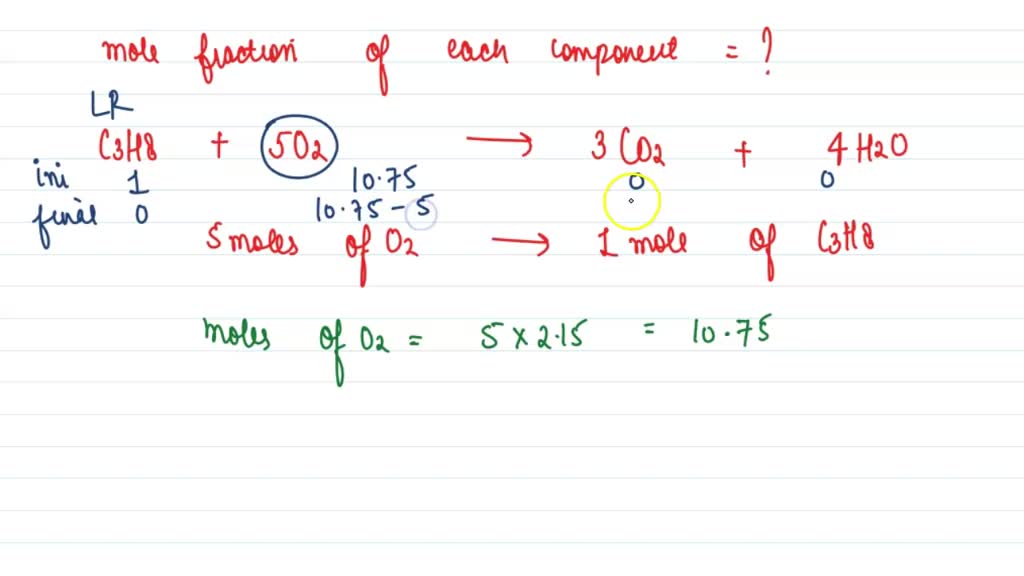
SOLVED 1. Interpretar la siguiente ecuación C3H8 + O2 → CO2 + H2O 2. 2H2(g) + 02(g) → 2H2O(l
Balance the reaction of C3H8 + O2 = CO + H2O using this chemical equation balancer! ChemicalAid. ⚛️. Carbonyl Carbonyl Ligand Monoxide Flue Gas Carbon Oxide [Co] C#O Co. CO Molar Mass CO Bond Polarity CO. For example, C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but XC2H5 + O2 = XOH + CO2 + H2O will. Compound states [like.
Balance the following equation C3H8 + O2 > CO2 + H2O
Latihan Soal Hukum Gay Lussac (Sedang) 1. Pertanyaan. Untuk membakar campuran gas etana (C2H6) ( C 2 H 6) dan gas propana (C3H8) ( C 3 H 8) diperlukan 18 18 L gas oksigen. Jika pada P dan T yang sama, dihasilkan 10, 5 10, 5 L gas karbon dioksida. Berapakah volume etana dan propana berturut-turut. . 1.

How to balance C3H8+O2=CO2+H2OChemical equation C3H8+O2=CO2+H2OReaction balance C3H8+O2=CO2
Be prepared with the most accurate 10-day forecast for Regimiento Buin, Santiago Metropolitan Region, Chile with highs, lows, chance of precipitation from The Weather Channel and Weather.com
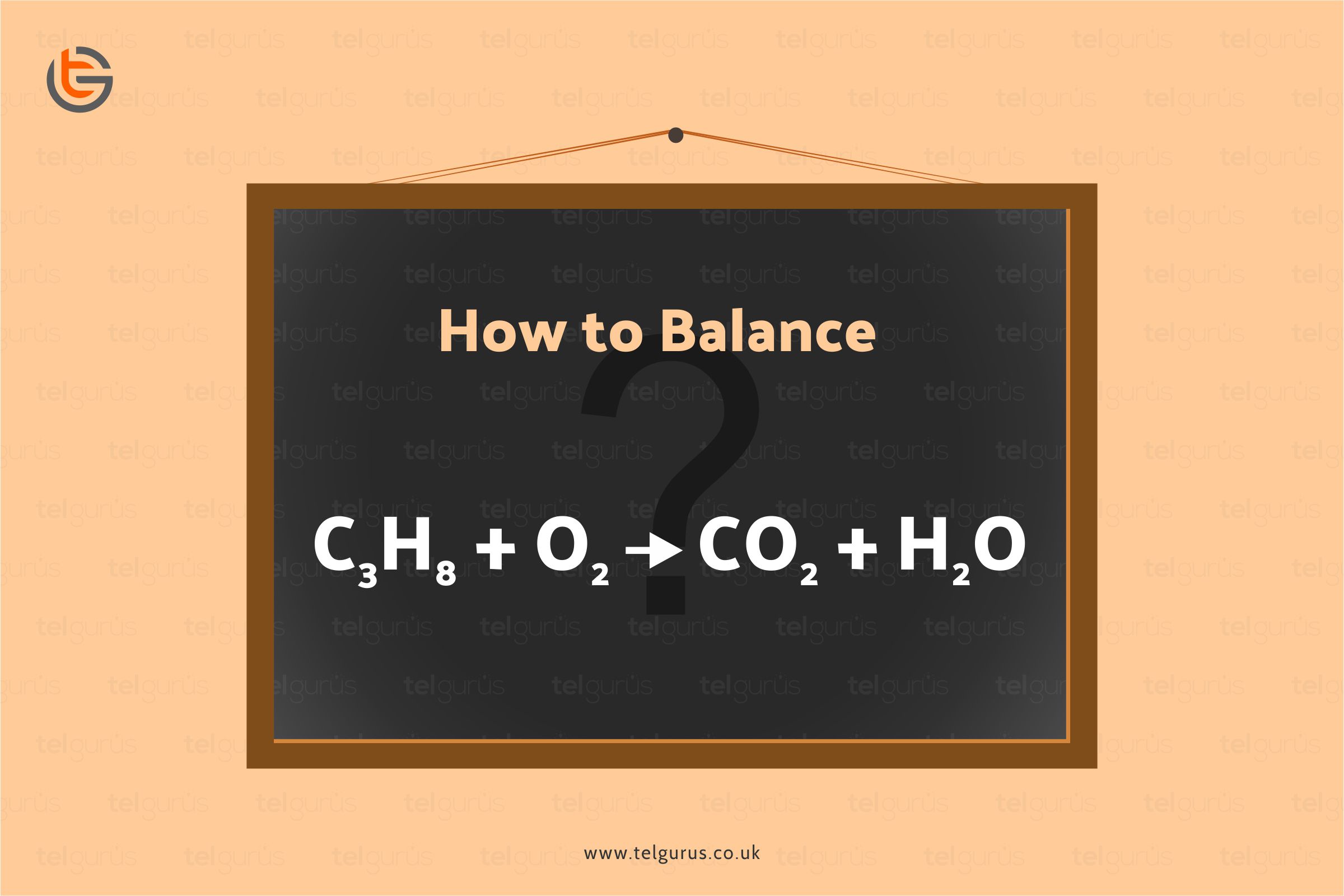
Balance the following equation C3H8 + O2 > CO2 + H2O
Word Equation. Propane + Dioxygen = Carbon Dioxide + Water + Carbon Dioxide. C3H8 + O2 = CO2 + H2O + CO2 is a Combustion reaction where one mole of Propane [C 3 H 8] and five moles of Dioxygen [O 2] react to form minus one moles of Carbon Dioxide [CO 2], four moles of Water [H 2 O] and four moles of Carbon Dioxide [CO 2] Show Chemical Structure.

Balance this equation C3H8 +O2 CO2+H2O Science Chemical Reactions and Equations
Salah satu cara untuk menyetarakan reaksi tersebut yaitu dengan menambahkan koefisien pada setiap senyawa. Kita asumsikan dulu ya koefisien C3H8 = 1, sehingga untuk koefisien senyawa yang lain dituliskan dengan huruf sebagai berikut: C3H8 + a O2 → b CO2 + c H2O - untuk C 1 x 3 = b (indeks C dikalikan koefisien) b = 3 - untuk H 1 x 8 = 2c c.

C3H8+O2 CO2+H2O Determinar los gramos de CO2 que producen en la combustion de 3,01x10^23
Now I multiply the CO2 by 3 to match the amount of C as on the LHS. C3H 8 + O2 → 3CO2 +4H 2O. The last step is to determine the coefficient of the oxygen gas on the LHS. We see that we have 6 +4 = 10 oxygen molecules on the RHS, so we will have 10 2 = 5 molecules on the LHS. The final balanced chemical equation is. C3H 8 + 5O2 → 3CO2 + 4H 2O.

Solved 5 3 4 11. C3H8 + O2 CO2 + H2O (Balance the equation
Balance the reaction of C3H8 + H2O = H2 + CO using this chemical equation balancer! ChemicalAid. ⚛️. (assuming constant volume in a closed system and no accumulation of intermediates or side products). C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but XC2H5 + O2 = XOH + CO2 + H2O will. Compound states [like (s) (aq) or (g.
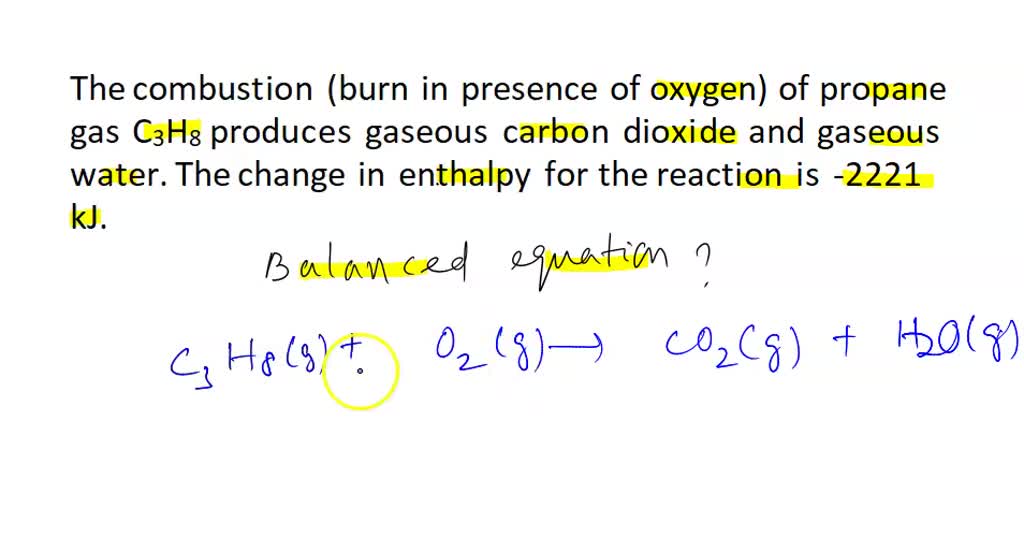
SOLVED A water heater is fueled by the combustion of propane as described by the following
Here are all the details of Buin available below. Buin Postal address. Carlos Condell Nº 415. Buin. Chile. Buin Phone number. (02) 8218400. International: +56 02 8218400. Buin Fax number.
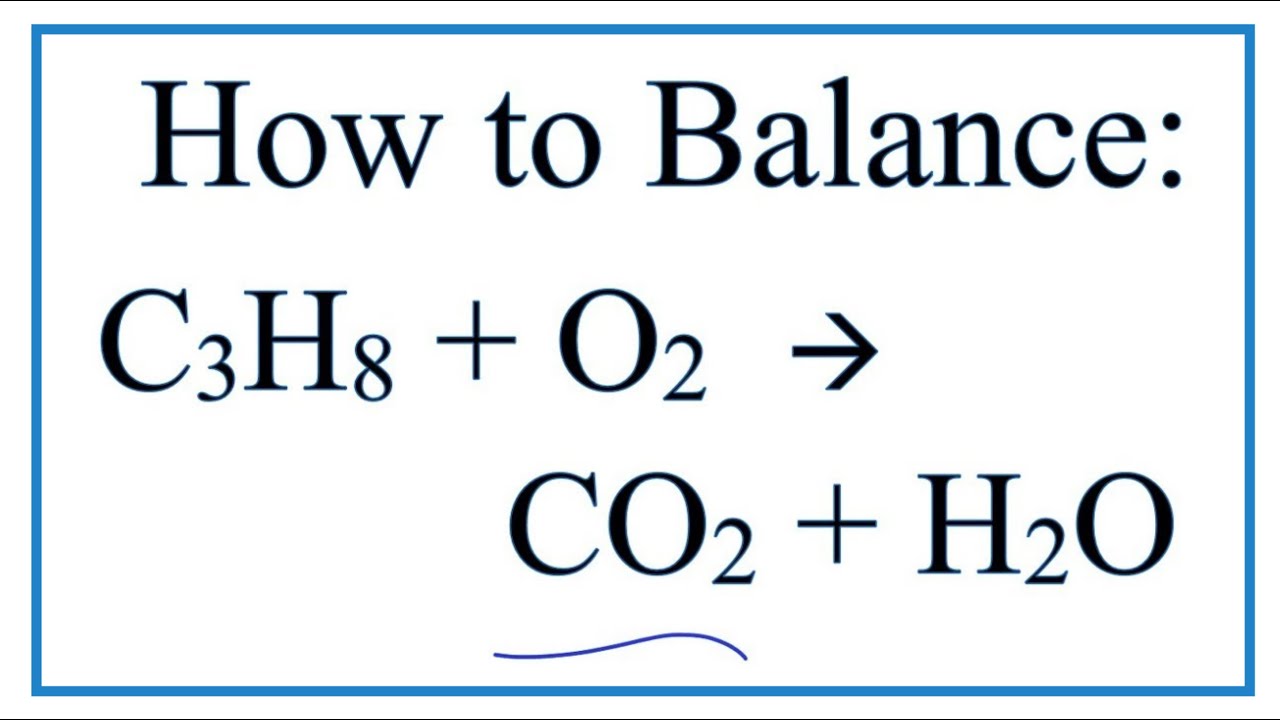
How to Balance C3H8 + O2 = CO2 + H2O (Propane Combustion Reaction) YouTube
If a spark is available, reaction of Propane and oxygen gases can be an explosive one. Propane (C3H8) is a gas at room temperature. When propane is burnt with oxygen (O2) gas, carbon dioxide (CO2) and water (H2O) are given as products. Heat is released from the combustion of propane.

Balancing the Equation C3H8 + O2 = CO2 + H2O (and Type of Reaction) YouTube
In this video we'll balance the equation C3H8 + O2 = CO2 + H2O and provide the correct coefficients for each compound.To balance C3H8 + O2 = CO2 + H2O you'll.