
PEMBUATAN LARUTAN BUFFER ASAM SITRAT Na2HPO4 PENDIDIKAN KIMIA 2016 UNIVERSITAS NEGERI PADANG
2.421 g. 0.0126 M. Prepare 800 mL of distilled water in a suitable container. Add 25.703 g of Sodium Citrate dihydrate to the solution. Add 2.421 g of Citric Acid to the solution. Adjust solution to final desired pH using HCl or NaOH. Add distilled water until the volume is 1 L. To make a purchase inquiry for this buffer, please provide your.

Facs Staining Buffer Recipe
Citric acid is an organic compound with the chemical formula HOC(CO 2 H)(CH 2 CO 2 H) 2. It is a colorless weak organic acid. It occurs naturally in citrus fruits.In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.. More than two million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a.

Jual Larutan Buffer Sitrat request pH 3.0 sampai 6.2/Bufer Kab. Bantul nitrakimia Tokopedia
Lampiran 1. Cara Pembuatan Buffer Sitrat Buffer sitrat dibuat dengan campuran larutan A yaitu larutan asam sitrat dan larutan B yaitu Na-sitrat, adapun ketentuan larutan yang digunakan : Larutan A : 0,1 M larutan asam sitrat (21,01 g dalam 1000 mL) Larutan B : 0,1 M larutan Na-sitrat (29,41 g C 6 H 5 O 7 Na 3 2H 2 O dalam 1000 mL)

BUFFER SOLUTIONS HOW TO MAKE BUFFER ? TYPES OF BUFFER HOW BUFFERS WORK ? YouTube
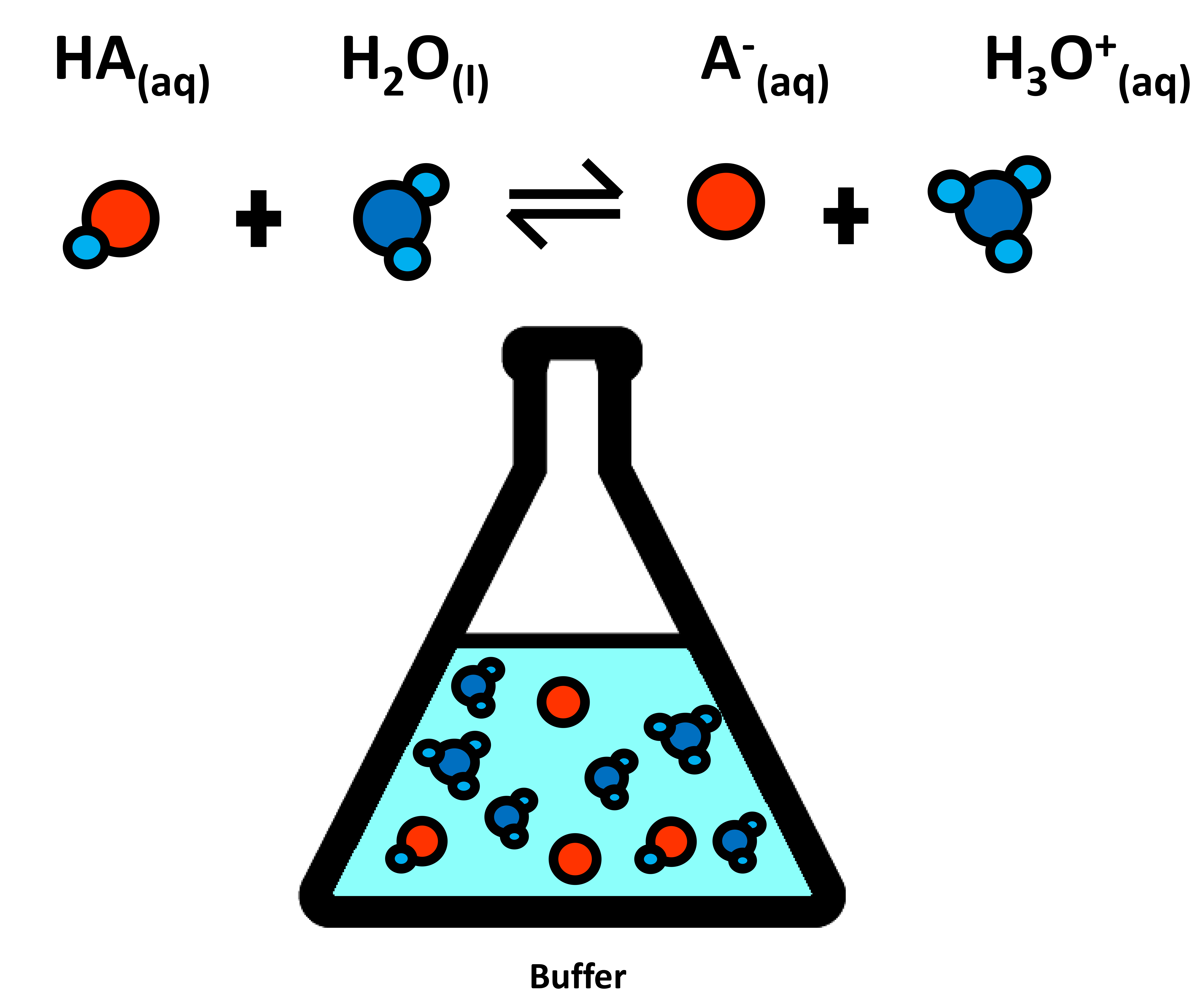
A buffer is a solution that can resist pH change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus maintaining the pH of the solution relatively stable. This is important for processes and/or reactions which require specific and stable pH ranges.
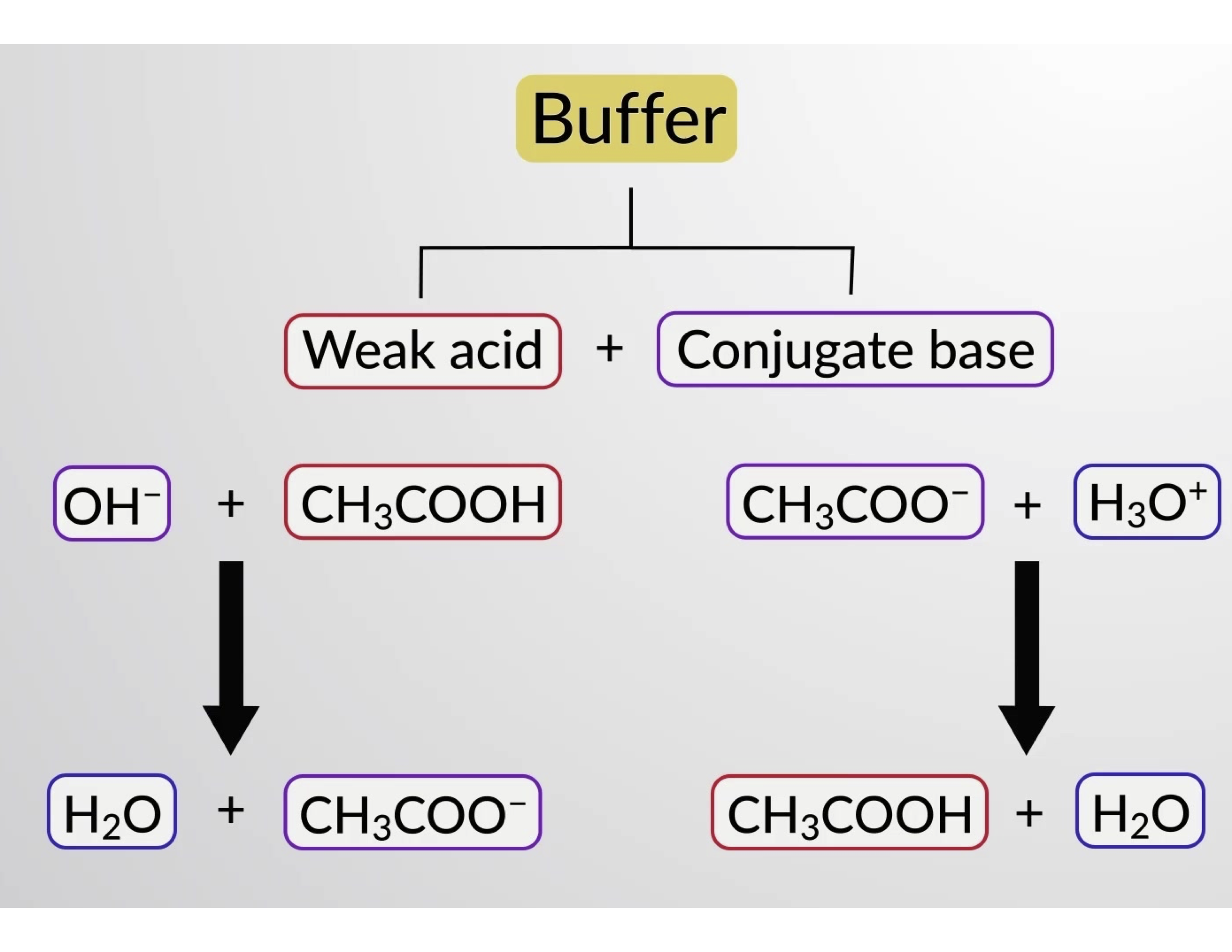
Buffers
Citric acid is a weak tricarboxylic acid found in citrus fruits like lemons, which contain 7-9% citric acid according to their dry weight. The three carboxylate groups of citric acid monohydrate have different pKa values, namely 3.15, 4.78, and 6.40 [ 7 ]. Until 1919, lemons were the main source of citric acid ( Figure 1 ).

(PDF) Pengaruh Aditif Dalam Larutan Watts Buffer Sitrat Terhadap Karakteristik Deposit Nikel
Buffer Calculations: Formula and Equations. Molar solution equation: desired molarity × formula weight × solution final volume (L) = grams needed Percentage by weight (w/v): (% buffer desired / 100) × final buffer volume (mL) = g of starting material needed Henderson-Hasselbach equation: pH = pKa + log [A-]/[HA] The Henderson-Hasselbalch equation enables determination of a buffer solution's.
Sodyum Sitrat 100 ml Flakon 1 Adet « Ozon
Potassium Chloride - KCl Oxalic Acid - C2H2O. 4. Sodium Oxalate - C2Na2O 4 Potassium Tetraxalate Maleic Acid - C4H4O. 4. Phosphoric Acid - H3PO. 4. Potassium Phosphate, Monobasic - H2KO4P Potassium Phosphate, Dibasic - HK2PO 4 Potassium Phosphate, Tribasic - K3PO4 Sodium Phosphate, Monobasic - H2NaPO. 4. Sodium Phosphate.

(DOC) PEMBUATAN BUFFER ASETAT, BUFFER FOSFAT dan BUFFER SITRAT Ervan Togatorop Academia.edu
Citrate-Phosphate Buffer (0.15 M, pH 5.0) preparation guide and recipe. Recipe can be automatically scaled by entering desired final volume. A traditional buffer originally introduced in 1921. Since the Citrate-Phosphate buffer (also known as the McIlvaine buffer) only has 2 ingredients, the recipe can be adjusted to a pH range of 3-8. It is used for multiple applications in cell biology,
PDF examples of buffer solutions PDF Télécharger Download
pH Ranges of Selected Biological Buffers Chart (25 °C, 0.1 M) Tris or Trizma ® Buffer Preparation - pH vs. Temperature. Phosphate Buffer Preparation - 0.2 M solution. Citric Acid - Na 2 HPO 4 Buffer Preparation, pH 2.6-7.6. Citric Acid - Sodium Citrate Buffer Preparation, pH 3.0-6.2. Sodium Acetate - Acetic Acid Buffer Preparation.

Cara Kerja Larutan Penyangga Asam dan Basa Kimia SMA Kelas 11 Materi Larutan Buffer YouTube
SAFETY DATA SHEET Revision Date 24-Feb-2020 Revision Number 2 1. Identification Product Name Citrate buffer, 0.2M buffer solution, pH 6.0, low endotoxin Cat No. : J67266 Synonyms No information available Recommended Use Laboratory chemicals. Uses advised against Food, drug, pesticide or biocidal product use. Details of the supplier of the safety data sheet

Prosedur Kerja Buffer Asam sitratNa2HPO4 YouTube
Find citrate buffer with 3.5 ph and related products for scientific research at MilliporeSigma. US EN. Products Applications Services Documents Support Advanced Search. Structure Search. Search Within. Products Building Blocks Explorer Technical Documents Site Content Papers Genes.

Coating Buffer Boca Scientific
Here are the recipes from IHC world. Sodium Citrate Buffer (10mM Sodium Citrate, 0.05% Tween 20, pH 6.0): Tri-sodium citrate (dihydrate) - 2.94 g. Distilled water - 1000 ml. Mix to dissolve.

Jual Asam Sitrat Citric Acid Monohydrate Analis Merck Per Gram Kota Free Hot Nude Porn Pic Gallery
Sodium citrate is the sodium salt of citric acid. It is white, crystalline powder or white, granular crystals, slightly deliquescent in moist air, freely soluble in water, practically insoluble in alcohol. Like citric acid, it has a sour taste. From the medical point of view, it is used as alkalinizing agent.

Praktikum Pembuatan Larutan Buffer Sitrat (C₆H₈O₇) YouTube
Two Options to Make the Buffer. There are two methods of making sodium citrate buffers, depending on the materials accessible to you. If you have both citric acid and the conjugate base, create a stock solution of each by mixing 21 grams of citric acid in 1 liter of distilled water, and 29.4 grams of sodium citrate in 1 liter of distilled water.

Percobaan Buffer Asam SitratNa2HPO4 YouTube
A buffer solution has the function of resisting changes in pH even when adding powerful acids or bases. However, in the physiological environment the buffered system also provides cofactors for enzymatic reactions, critical salts and even essential nutrients for cells and tissues.

Jual Larutan Buffer Sitrat request pH 3.0 sampai 6.2/Bufer Kab. Bantul nitrakimia Tokopedia
Buffer sitrat berfungsi untuk menjaga pH makanan olahan dalam kaleng agar tidak tidak mudah rusak atau teroksidasi. Hal ini sesuai dengan pernyataan hannato (2014) yang menyatakan bahwa umumnya larutan penyangga berfungsi untuk menjaga keseimbangan pH pada suatu produk pangan. V. KESIMPULAN DAN SARAN V.1 Kesimpulan Kesimpulan dari praktikum.