
Describe Bohr’s model of the hydrogen atom. bitWise Academy
With sodium, however, we observe a yellow color because the most intense lines in its spectrum are in the yellow portion of the spectrum, at about 589 nm. Figure 7.3.1: The Emission of Light by Hydrogen Atoms. (a) A sample of excited hydrogen atoms emits a characteristic red light.
35 Gambar Struktur Atom Lengkap dengan Konfigurasi Elektron dan Diagram Orbitalnya MateriKimia
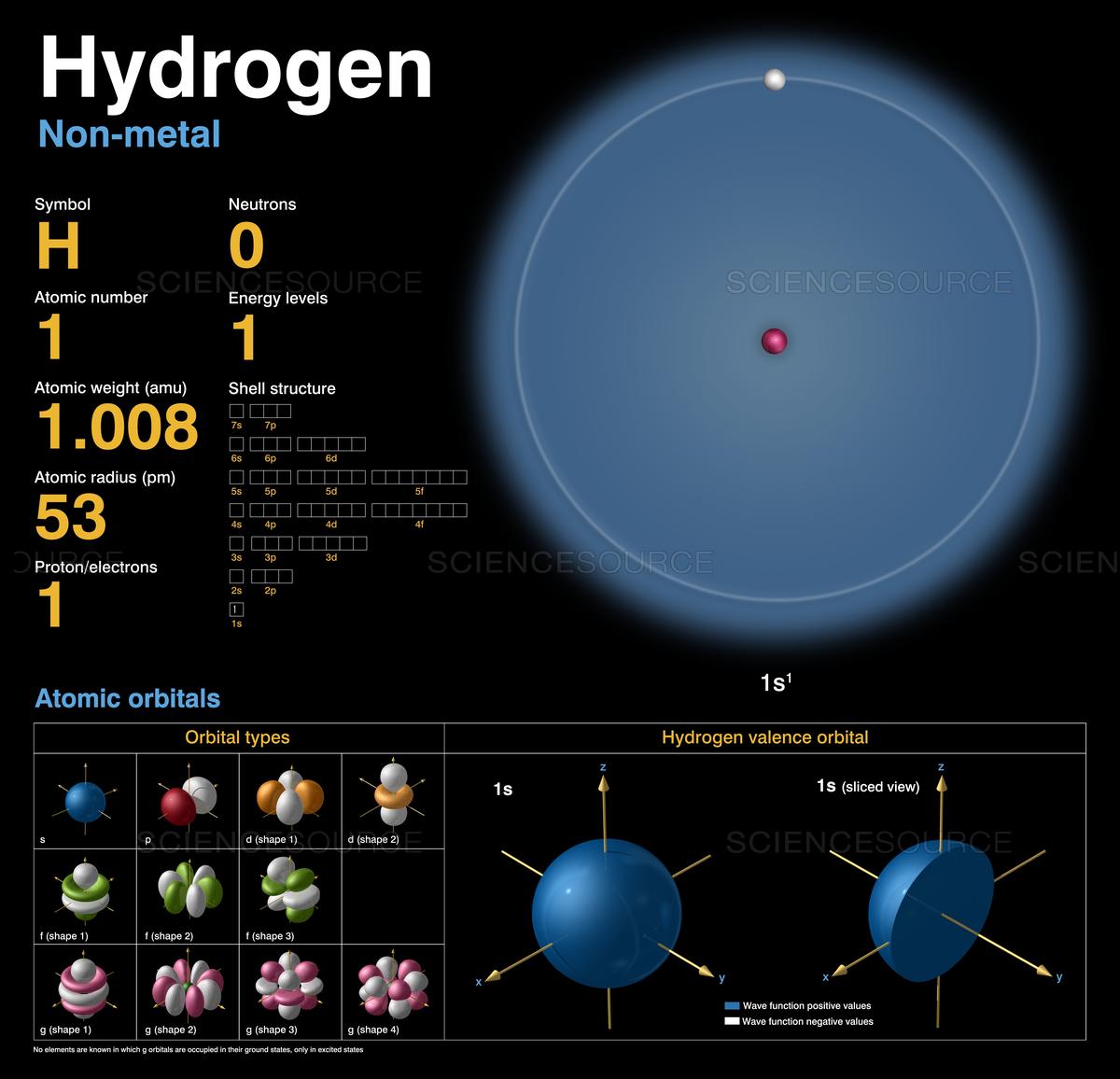
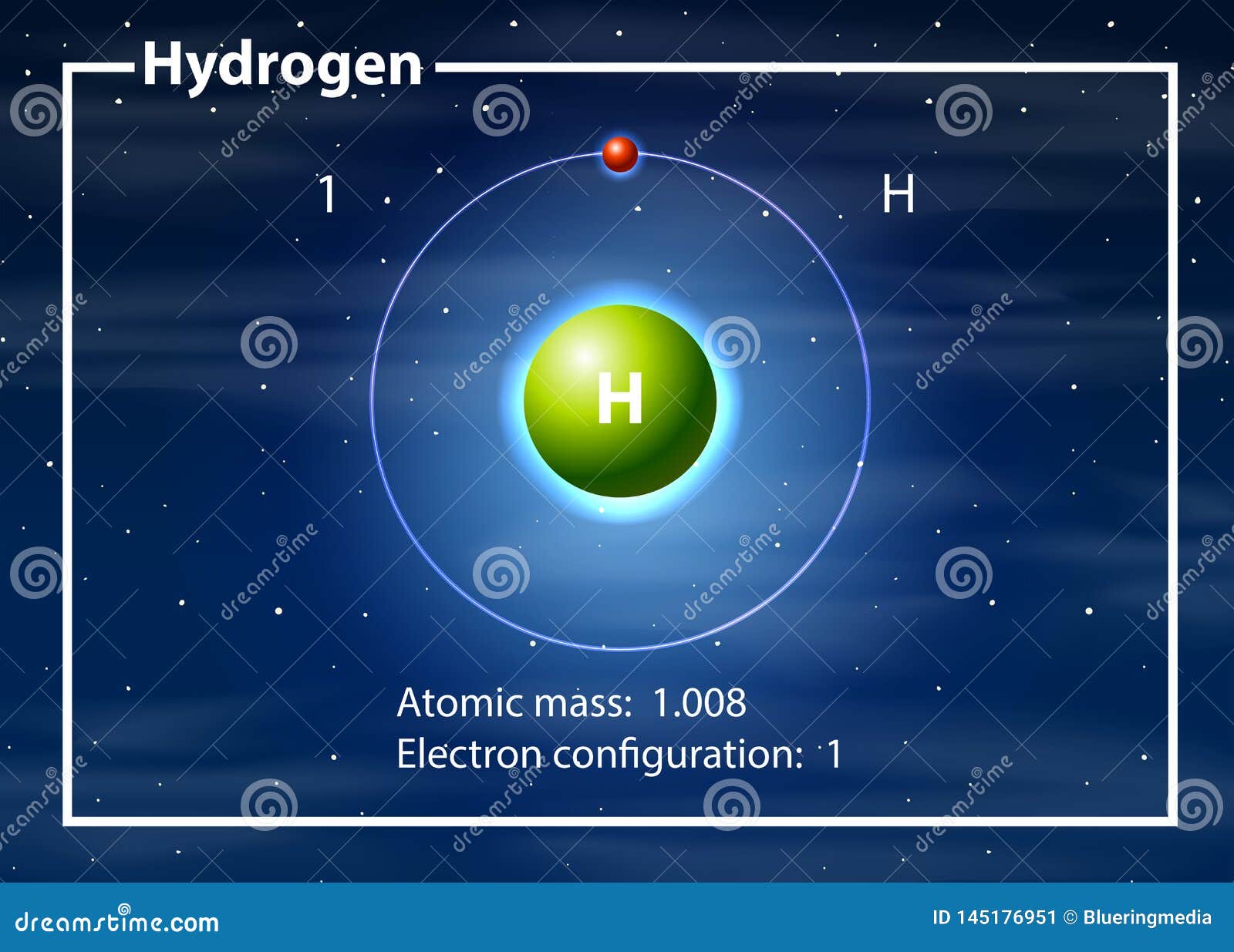
Hydrogen is a chemical element; it has symbol H and atomic number 1. It is the lightest element and, at standard conditions, is a gas of diatomic molecules with the formula H2, sometimes called dihydrogen, [10] but more commonly called hydrogen gas, molecular hydrogen or simply hydrogen. It is colorless, odorless, tasteless, [11] non-toxic, and.

Hydrogen atom diagram concept illustration Stock Vector Image & Art Alamy
Bohr's model calculated the following energies for an electron in the shell, n. . : E ( n) = − 1 n 2 ⋅ 13.6 eV. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the photon energy is. h ν = Δ E = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 eV.

Diagram representation element hydrogen Royalty Free Vector
Atomic Number: 1. Hydrogen is the first element in the periodic table, meaning it has an atomic number of 1 or 1 proton in each hydrogen atom. The name of the element comes from the Greek words hydro for "water" and genes for "forming," since hydrogen bonds with oxygen to form water (H 2 O). Robert Boyle produced hydrogen gas in 1671 during an.

Diagram Representation Of The Element Hydrogen Stock Vector Image 59013305
In this chapter we will consider the hydrogen atom as a proton fixed at the origin, orbited by an electron of reduced mass μ. The potential due to electrostatic attraction is: V(r) = − e2 4πε0r, where ε0 is the constant permittivity of vacuum. The kinetic energy term in the Hamiltonian is.
Render Atom Structure Hydrogen Isolated White Backgroun Stock Photo by ©oorka5 245708802
The hydrogen atom is one of the few real physical systems for which the allowed quantum states of a particle and corresponding energies can be solved for exactly (as opposed to approximately) in non-relativistic quantum mechanics. In the most basic quantum mechanical model of hydrogen, the proton is taken to be a fixed source of an electric potential and the Schrödinger equation for the.

Hydrogen atom on white background Royalty Free Vector Image
Model Atom Hidrogen - Mekanika Kuantum, Atom Hidrogen, Model Bohr - PhET. Lompat ke Isi Utama.
Atoms & Molecules echapter — The Biology Primer
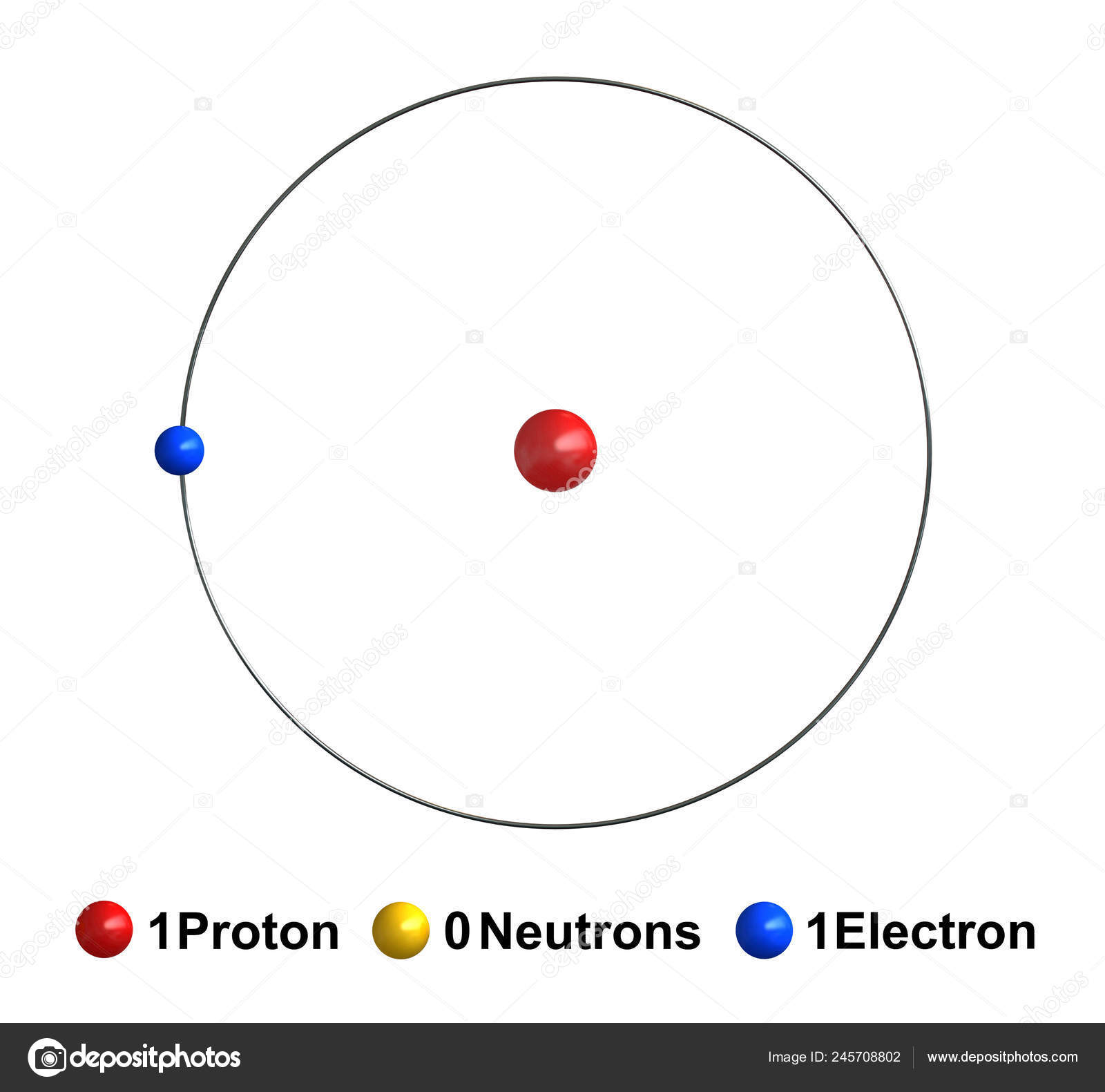
hydrogen, Lightest chemical element, chemical symbol H, atomic number 1.A colourless, odourless, tasteless, flammable gas, it occurs as the diatomic molecule H 2.Its atom consists of one proton (the nucleus) and one electron; the isotopes deuterium and tritium have an additional one and two nuclear neutrons, respectively. Though only the ninth most abundant element on Earth, it represents.

Átomo de hidrógeno en el fondo blanco
The hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. The hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton ().In Bohr's model, the electron is pulled around the proton in a perfectly circular orbit by an attractive Coulomb force.

Bohr model scientific hydrogen atom Royalty Free Vector
The electron's speed is largest in the first Bohr orbit, for n = 1, which is the orbit closest to the nucleus. The radius of the first Bohr orbit is called the Bohr radius of hydrogen, denoted as a0. Its value is obtained by setting n = 1 in Equation 6.5.6: a0 = 4πϵ0 ℏ2 mee2 = 5.29 × 10 − 11m = 0.529 Å.

Hydrogen Definition, Structure, Properties & Uses Embibe
Atomic hydrogen may be generated in aqueous solution through the solvation of electrons. e−(aq) +H3O+ ⇋ H. +H2O (2.4.3) (2.4.3) e ( a q) − + H 3 O + ⇋ H. + H 2 O. The formation equilibrium constant (K eq) is very small resulting in very low concentrations being generated (10 -5 M). As expected solvated atomic hydrogen is a strong.

Hydrogen Atom · Free vector graphic on Pixabay
where h f h f is the energy of either an emitted or an absorbed photon with frequency f.The second quantization condition states that an electron's change in energy in the hydrogen atom is quantized. These three postulates of the early quantum theory of the hydrogen atom allow us to derive not only the Rydberg formula, but also the value of the Rydberg constant and other important properties.

Bohr Atomic Model Of Hydrogen
The hydrogen atom consists of an electron and a proton bound together by the attractive electrostatic force between the negative and positive charges of these particles. Our experience with the one-dimensional particle in a box shows that a spatially restricted particle takes on only discrete values of the total energy. This conclusion carries.

Hydrogen Boundless Chemistry
Global hydrogen production by technology in the Net Zero Scenario, 2019-2030. IEA. Licence: CC BY 4.0. Dedicated hydrogen production today is primarily based on fossil fuel technologies, with around a sixth of the global hydrogen supply coming from "by-product" hydrogen, mainly in the petrochemical industry.
Hydrogen Atom Diagram Concept Stock Vector Illustration of abstract, school 145176951
Try out different models by shooting light at the atom. Check how the prediction of the model matches the experimental results. How did scientists figure out the structure of atoms without looking at them? Try out different models by shooting light at the atom. Check how the prediction of the model matches the experimental results.

How to Learn About the Chemistry of the Hydrogen Atom 12 Steps
The mass of an atom relative to that of carbon-12. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, the value given is the abundance weighted average. Isotopes Atoms of the same element with different numbers of neutrons. CAS number