
Amlodipine 5mg Tablets 28 Tablets
Amlodipine is an oral dihydropyridine calcium channel blocker. Amlodipine works by blocking the voltage-dependent L-type calcium channels, thereby inhibiting the initial influx of calcium. Compared to nifedipine and other medications in the dihydropyridine class, amlodipine has the longest half-life at 30 to 50 hours. The benefit of such a long half-life is the ability to have once-daily.
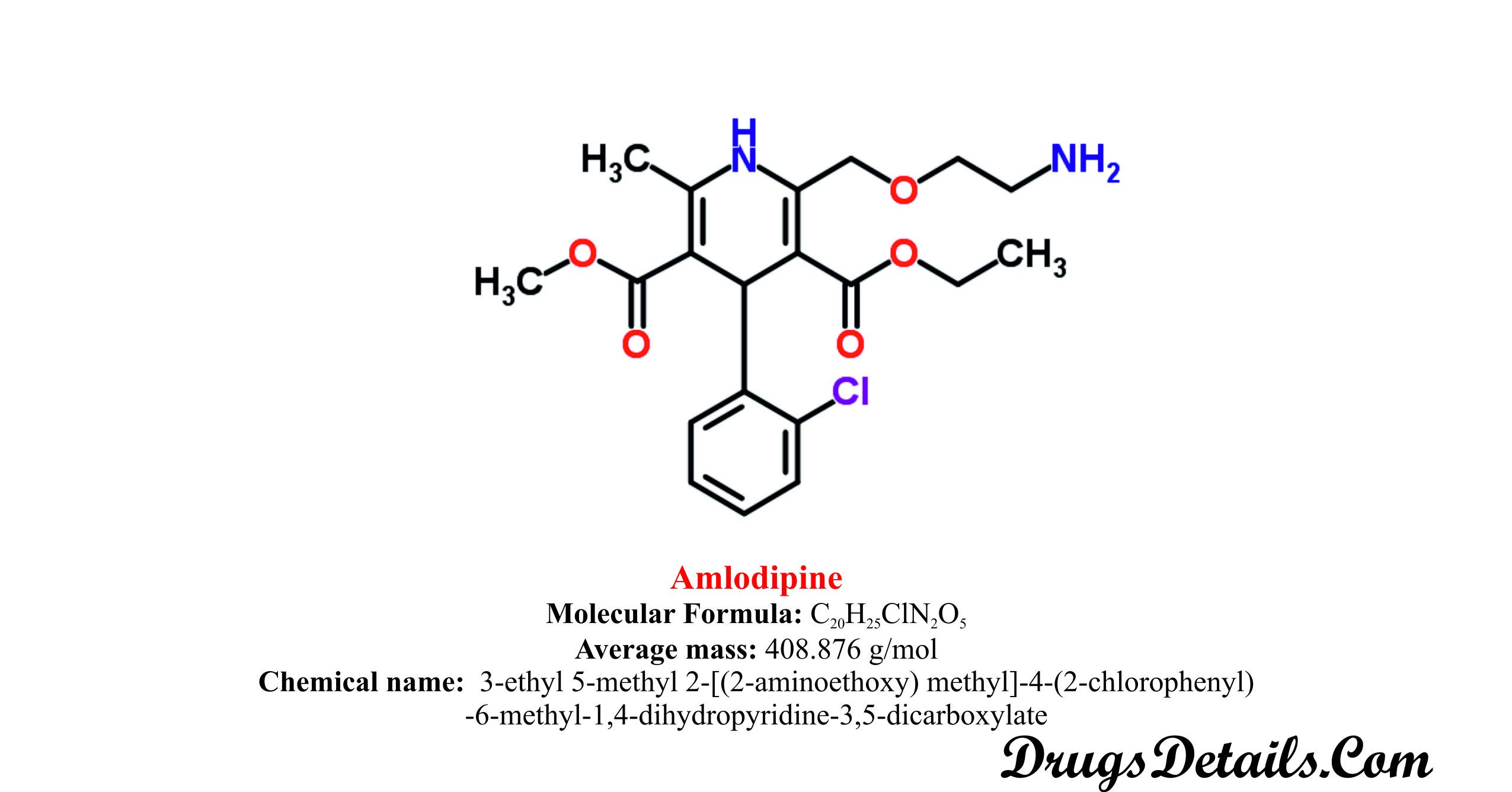
Amlodipine Drug Details
EP3 960 158A1 2 5 10 15 20 25 30 35 40 45 50 55 Description CROSS-REFERENCE [0001] This application claims the benefit of U. S. Application Serial No. 62/405,455 filed October 7, 2016, which is

AMLODIPIN besilat AL 5 mg Tabletten 100 St
United States. Dates. Priority: 2021/02/01. Grant: 2022/02/22. Description. This web page summarizes information in PubChem about patent US-11253474-B1. This includes chemicals mentioned, as reported by PubChem contributors, as well as other content, such as title, abstract, and International Patent Classification (IPC) codes.

Amlodipin 1 A Pharma® 5 mg N 100 St
Justia Patents US Patent for Pharmaceutical solution of amlodipine Patent (Patent # 11,458,095) Pharmaceutical solution of amlodipine . Jan 14, 2022 - LIQMEDS WORLDWIDE LIMITED. Disclosed herein is a liquid pharmaceutical formulation substantially free of water, comprising: (i) amlodipine or a pharmaceutically acceptable salt thereof, (ii) at.

Patent EP1687273A1 Process for preparation of chiral amlodipine salts Google Patents
Page. Patient Forums for Amlodipine. Part of the Heart Health category. Symptom, treatment and advice from community members.

Amlodipine Tablet Uses, Side Effects, Dosage and Price
Justia Patents US Patent Application for FIXED DOSE COMBINATION OF TELMISARTAN, HYDROCHLOROTHIAZIDE AND AMLODIPINE Patent Application (Application #20200316029) FIXED DOSE COMBINATION OF TELMISARTAN, HYDROCHLOROTHIAZIDE AND AMLODIPINE . May 24, 2017. The present invention discloses a pharmaceutical tablet with a first layer comprising.

US6653481B2 Process for making amlodipine Google Patents
Amlodipine and related analogues thereof are prepared by the following general reaction scheme: R 1 and R 2 each independently represent a C 1 -C 4 alkyl group. The process provides for the formation of compounds of formula (1) in good yield and purity. Further, the compounds of formula (1) can be used as calcium channel blockers or as reference standards or reference markers for checking the.

Patent US6653481 Process for making amlodipine Google Patents
10. The tablet according to claim 1, wherein the first layer of telmisartan is produced by spray-drying an aqueous solution comprising telmisartan and a basic agent to obtain a spray-dried granulate, mixing the spray-dried granulate with a water-soluble diluent to obtain a premix, mixing the premix with a lubricant to obtain a final blend, and compressing the final blend to form the first.

Amlodipine Mylan Uses, Dosage, Side Effects, Precautions & Warnings
Amlodipine, sold under the brand name Norvasc among others, is a calcium channel blocker medication used to treat high blood pressure, coronary artery disease (CAD) and variant angina (also called Prinzmetal angina or coronary artery vasospasm, among other names). It is taken orally (swallowed by mouth).. Common side effects include swelling, feeling tired, abdominal pain, and nausea.
Amlodipin Stada 10 mg Tabletten
Justia Patents At 3-position US Patent Application for Aspartate derivative of amlodipine Patent Application (Application #20020128296) Aspartate derivative of amlodipine . Aug 27, 2001. An amlodipine derivative having the following formula is useful, either alone or in combination with amlodipine, as a pharmaceutical in treating angina and.
Amlodipine Tablets 90/Bottle McGuff Medical Products
The pharmaceutical composition is stable, that is, after storage for about 3 months at about 40°C at about 75% relative humidity, it contains no more than 2.5% of (s,s)-diacid by weight relative to the benazepril and/or no more than 0.3% of impurity D by weight relative to the amlodipine.
INTERMEDIATE FOR THE SYNTHESIS OF AMLODIPINE, PREPARATION PROCESS AND CORRESPONDING UTILIZATION
The bilayer tablet strengths are 10 mg/320 gm and 5 mg/320 mg of amlodipine besylate and valsartan. [0023] In the pharmaceutical composition according to the invention, the active agent (s) comprises valsartan which includes its pharmaceutically acceptable salts, hydrates, solvates or polymorphs or enantiomers and racemates thereof in amount of.
What is Amlodipine uses, benefits and side effects Echo Pharmacy
A sterile aqueous solution comprising the besylate salt of amlodipine for parenteral administration. 10. A sterile aqueous solution as claimed in claim 9 comprising from 10 to 40% w/v of propylene glycol. 11. A sterile aqueous solution as claimed in claim 9 or claim 10 comprising about 1 % w/v sodium chloride. 12.

Amlodipin 1 A Pharma® 10 mg Tabletten N 100 St
Amlodipine is used alone or in combination with other medications to treat high blood pressure in adults and children 6 years and older. It is also used to treat certain types of angina (chest pain) and coronary artery disease (narrowing of the blood vessels that supply blood to the heart). Amlodipine is in a class of medications called calcium.

Patent EP1287826A1 A crystalline form of the free base of Amlodipine Google Patents
Generic name: amlodipine [ am-LOE-di-peen ] Brand names: Katerzia, Norliqva, Norvasc Dosage forms: oral tablet 5 mg, 10mg, 2.5 mg, oral liquid 1 mg/mL, oral suspension 1 mg/mL Drug class: Calcium channel blocking agents Medically reviewed by Melisa Puckey, BPharm.Last updated on Oct 29, 2023. Uses; Side effects; Warnings; Before taking; Dosage

EP1287826A1 A crystalline form of the free base of Amlodipine Google Patents
CROSS-REFERENCE. This application is a continuation of U.S. application Ser. No. 17/067,396 filed Oct. 9, 2020 which is a continuation of U.S. application Ser. No. 16/381,575 filed Apr. 11, 2019 which is continuation of International Application No. PCT/U2019/027044 filed Apr. 11, 2019 which claims priority to U.S. Provisional Application No. 62/656,188, filed Apr. 11, 2018, which is hereby.