
8HNO3 + 3Cu = 3Cu(NO3)2 + 2NO + 4H2O Calcula el volumen del Oxido Nitroso YouTube
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: What element is oxidized in the following chemical reaction? 3Cu + 8HNO3 → 3Cu (NO3)2 + 2NO + 4H20 oo ON OH Cu I have no idea. This answer is incorrect. Do not select this one!

3. 3Cu + 8HNO3 → 3Cu(NO3)2 + 2NO + 4H2Odenklemine göre, 6 mol Cu elementi 32 mol HNO3 asidiyle
Since there is an equal number of each element in the reactants and products of 3CuS + 8HNO3 = 3CuSO4 + 8NO + 4H2O, the equation is balanced. Balance CuS + HNO3 = CuSO4 + NO + H2O Using Inspection The law of conservation of mass states that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a.

3Cu(k) + 8HNO3(suda) > 3Cu(NO3)2(suda) + 2NO(g) + 4H2O(s) tepkimesiyle ilgili hangileri
For those that are redox reactions, identify the oxidizing and reducing agents. 3Cu + 8HNO3 → 3Cu(NO3)2 + 2NO + 4H2O 2C2H6 + 7O2 → 4CO2 + 6H2O 2NaHCO3 → Na2CO3 + CO2 + H2O 2K + 2H2O → 2KOH + H2. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
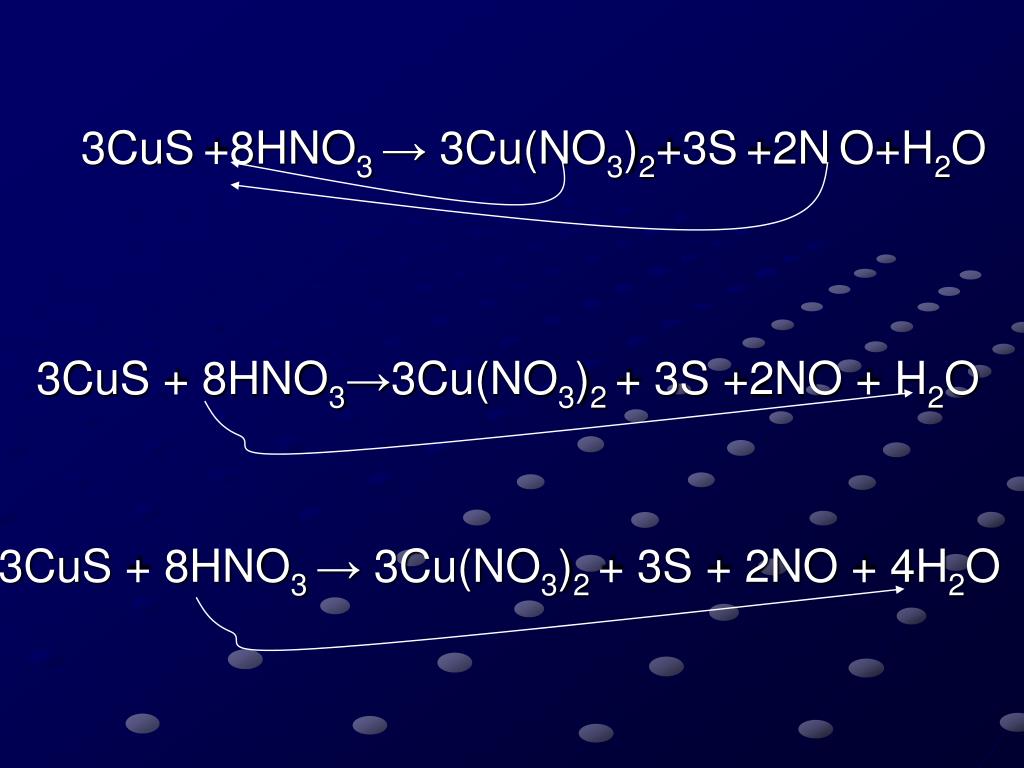
PPT CuS+HNO 3 →Cu(NO 3 ) 2 + S + NO+H 2 O PowerPoint Presentation, free download ID4894395
Final answer: In the given chemical reaction, the element oxidized and the reducing agent is Copper (Cu). The number of electrons transferred from the reducing agent to the oxidizing agent is six. Explanation: In the reaction equation, 3Cu(s) + 8HNO3(aq) --> 2NO(g) + 3Cu(NO3)2(aq) + 4H2O(l), the element oxidized is Cu (Copper), as Copper goes from an oxidation state of 0 in Cu(s) to +2 in Cu.
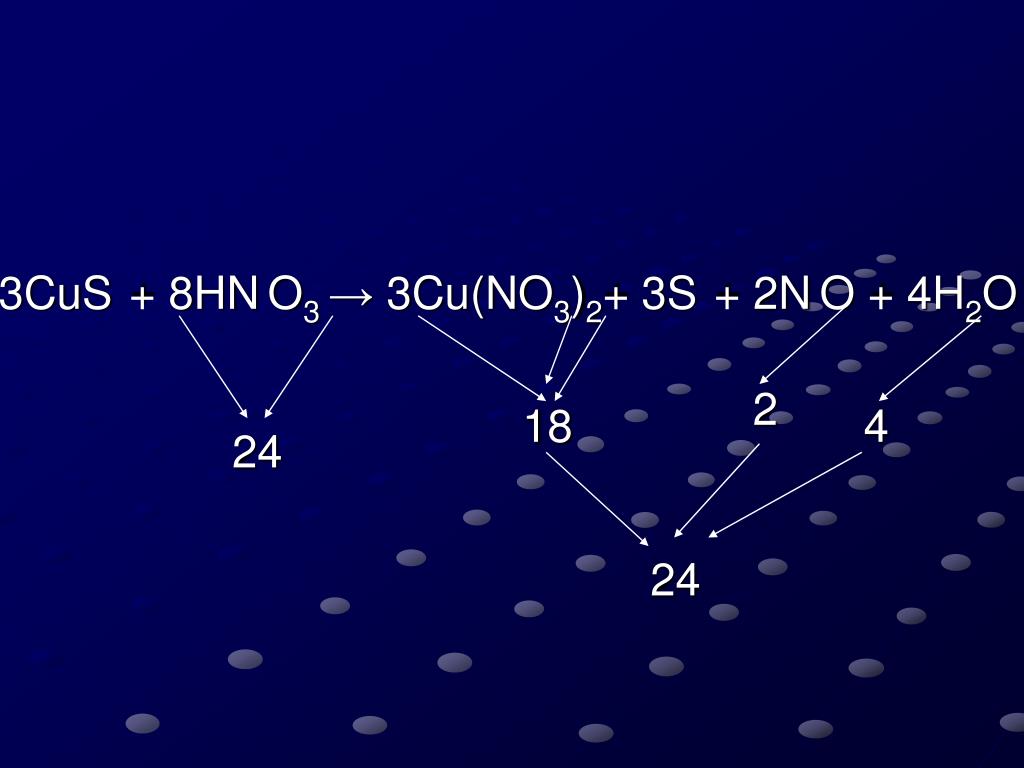
PPT CuS+HNO 3 →Cu(NO 3 ) 2 + S + NO+H 2 O PowerPoint Presentation, free download ID4894395
Since there is an equal number of each element in the reactants and products of 3CuS + 8HNO3 = 3Cu(NO3)2 + 3S + 2NO + 4H2O, the equation is balanced. Balance CuS + HNO3 = Cu(NO3)2 + S + NO + H2O Using Inspection. Add 2 molecules of Cu(NO 3) 2 to the product (right-hand) side to balance Nitrogen: CuS + 8 HNO 3 = 3 Cu(NO 3) 2 + S + 2 NO + 4 H 2 O.
[Solved] 3 Cu + 8HNO3 > 3 Cu(NO3)2 + 2 NO + 4 H20 In the above equation... Course Hero
In the reaction, 3Cu +8H N O3 → 3Cu(N O3)2 +2N O+4H 2O, copper atom loses two electrons to form Cu(I I) ion. In this process, it changes its oxidation state from 0 to +2. Thus, copper is oxidized. During the reaction, nitrogen present in nitric acid changes its oxidation state from +5 to +2. Thus, it is reduced.

Cu + 8 HNO3 3 Cu(NO3)2 + 2 NO + 4 H2O a) Determinar el número de moles de 3Cu (NO3)2 producidos
3Cu2S + 8HNO3 → 3CuS + 3Cu(NO3)2 + 2NO↑ + 4H2O - You-iggy

3Cu(k) + 8HNO3(suda) > 3Cu(NO3)2(suda) + 2NO(g) + 4H2O(s) tepkimesiyle ilgili hangileri
This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Consider the following balanced chemical equation: 3Cu (s) + 8HNO3 (aq) + 3Cu (NO3)2 (aq) + 4H₂O () + 2NO (g) If 4.42 moles of Cu (s) is reacted with 9.06 moles of HNO3 (aq), what is the limiting reagent? O.

Actividad de química De acuerdo con la ecuación.3Cu + 8HNO3 > 3 Cu(NO3)2 + 2NO + 4H2O
3 Cu +2 S-2 + 8 H +1 N +5 O-2 3 → 3 S 0 + 2 N +2 O-2 + 3Cu(NO 3) 2 + 4H 2 O. Finally, always check to see that the equation is balanced. First, verify that the equation contains the same type and number of atoms on both sides of the equation. ELEMENT LEFT. 3CuS + 8HNO 3 → 3Cu(NO 3) 2 + 3S + 2NO + 4H 2 O.
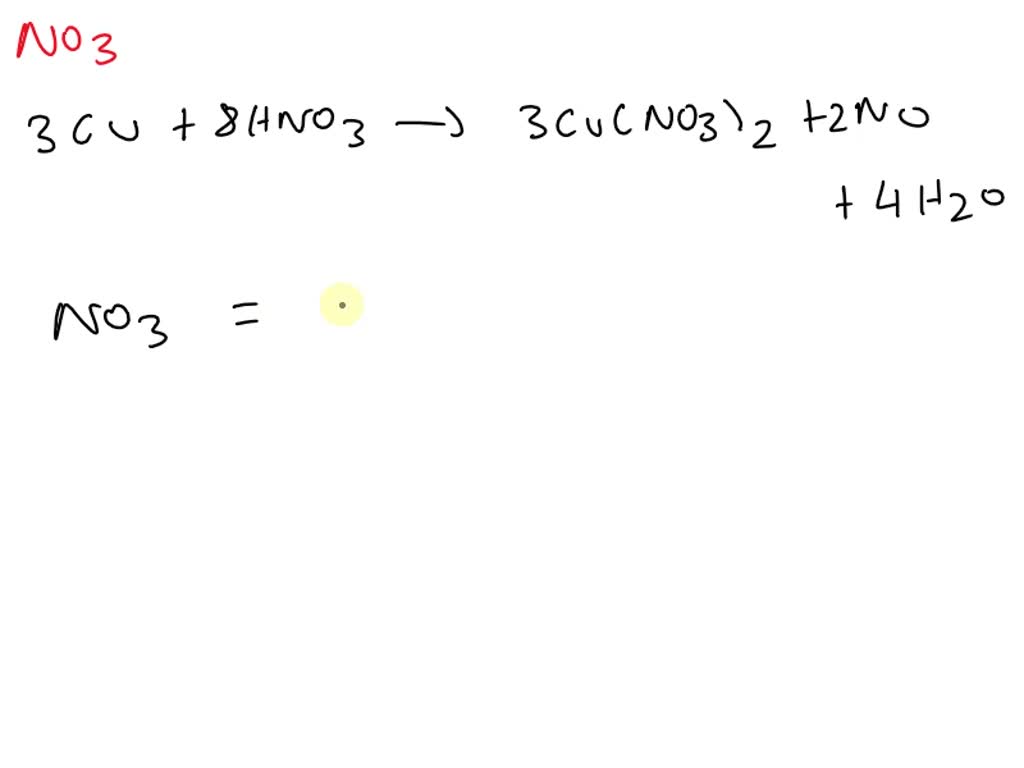
SOLVED For the equation 3Cu + 8HNO3 → 3Cu(NO3)2 + 2NO + 4H2O, how many units of NO3 are
Identify the atoms that are oxidized and reduced, the change in oxidation state for each, and the oxidizing and reducing agents in each of the following equa.

3Cu+8HNO3 [ok var arada] 3Cu[NO3]2+2x + 4H2O SORU X'İN FORMÜLÜ NEDİR? (Teşekkürler) (10
Search equation . Please enter the reactant or product to start the search

The equivalent weight of HNO3 (molecular weight = 63 ) in the following reaction is 3Cu + 8HNO3
Ana A. 18 April 2022 08:01. Perhatikan persamaan reaksi berikut! 3CuS (aq) + 8HNO3 (aq) → 3Cu (NO3)2 (aq) + 2NO (g) +3S (s) + 4H2O (l) pernyataan yang tepat mengenai persamaan reaksi tersebut adalah. a. CuS berperan sebagai oksidator, sedangkan HNO3 berperan sebagai reduktor. b. Atom N mengalami reduksi karena bilangan oksidasinya turun.
[Solved] 3 Cu + 8HNO3 g 3 Cu(NO3)2 + 2 NO + 4... Course Hero
There are 4 easy steps to find the molar mass of 3Cu + 8HNO3 + 3Cu(NO3)2 + 2NO + 4H2O based on its chemical formula. 1. Count The Number of Each Atom. The first step to finding the molar mass is to count the number of each atom present in a single molecule using the chemical formula, 3Cu + 8HNO3 + 3Cu(NO3)2 + 2NO + 4H2O:

For the equation 3cu 8hno3 → 3cu(no3)2 2no 4h2o, how many units of no3 are represented on the
Which statement about the following equation is correct? 3Cu + 8HNO 3 ® 3Cu (NO 3) 2 + 2NO + 4H 2 O. a) Nitrogen is reduced, and copper is oxidized. b) Nitrogen is oxidized, and copper is oxidized. c) Nitrogen is reduced, and oxygen is oxidized. d) Nitrogen is oxidized, and oxygen is oxidized. There are 4 steps to solve this one.

2. From the equation,3Cu + 8HNO3 → 3Cu (NO3)2 + 4H2O + 2NO(At. mass Cu = 64, H = 1, N = 14,0
3 Cu + 8HNO 3 → 3 Cu(NO 3) 2 + 2 NO + 4 H 2 O. In the above equation. 3 Cu + 8HNO3 → 3 Cu(NO3)2 + 2 NO + 4 H2O. In the above equation, how many grams of water can be made when 14.3 moles of HNO3 are consumed? Round your answer to the nearest tenth. given: 14.3 mol HNO3.
[Solved] 4 pts 3 Cu + 8HNO3 3 Cu(NO3)2 + 2 NO + 4 H20 In the above... Course Hero
Since there is an equal number of each element in the reactants and products of 3Cu + 8HNO3 = 3Cu(NO3)2 + 2NO + 4H2O, the equation is balanced. Balance Cu + HNO3 = Cu(NO3)2 + NO + H2O Using Inspection. Add 2 molecules of Cu(NO 3) 2 to the product (right-hand) side to balance Nitrogen: Cu + 8 HNO 3 = 3 Cu(NO 3) 2 + 2 NO + 4 H 2 O.