2 Butene Alchetron, The Free Social Encyclopedia
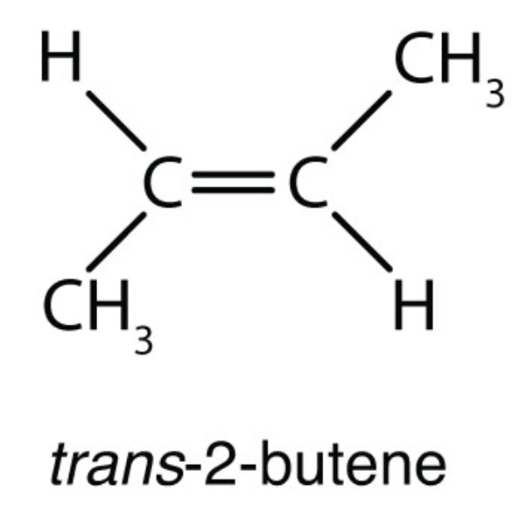
Structural Formula. C 4 H 8. trans-2-butene
[Solved] When 2methyl2butene reacts with aqueous bromine, the major... Course Hero
Figure 10.4a Addition reaction. The reaction between C=C double bond and bromine (Br 2) can be used as a test for the presence of alkene in an unknown sample. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. When bromine is added to the sample, if the reddish color disappear, that means the sample does.
[Solved] Cis2butene reacts with Br2 according to the scheme shown below.... Course Hero
1. High temperatures lead to radical reactions. ClX2/CClX4 C l X 2 / C C l X 4 is a standard addition reaction leading to vicinal dihalides, no high temp here. - Safdar Faisal. Apr 12, 2021 at 13:34.
[Solved] help with the following Draw product that Form 2 methyl2buten... Course Hero
Question: Draw the product obtained when trans-2-butene. Draw the product obtained when trans-2-butene is treated first with Br2 in CH2Cl2, second with NaNH2 in NH3, and then finally with Li in NH3. Please show each step. There are 2 steps to solve this one.

Alkene + Br2 + H2O YouTube
Electrophilic addition of HBr H B r to an alkene: Step 1 is an acid-base reaction: the π π electrons of the alkene act as a base and extract the acidic proton of HBr H B r. This leaves one of the carbons with a new bond to hydrogen, and the other with an incomplete octet and a positive formal charge. In step 2, the nucleophilic bromide anion.
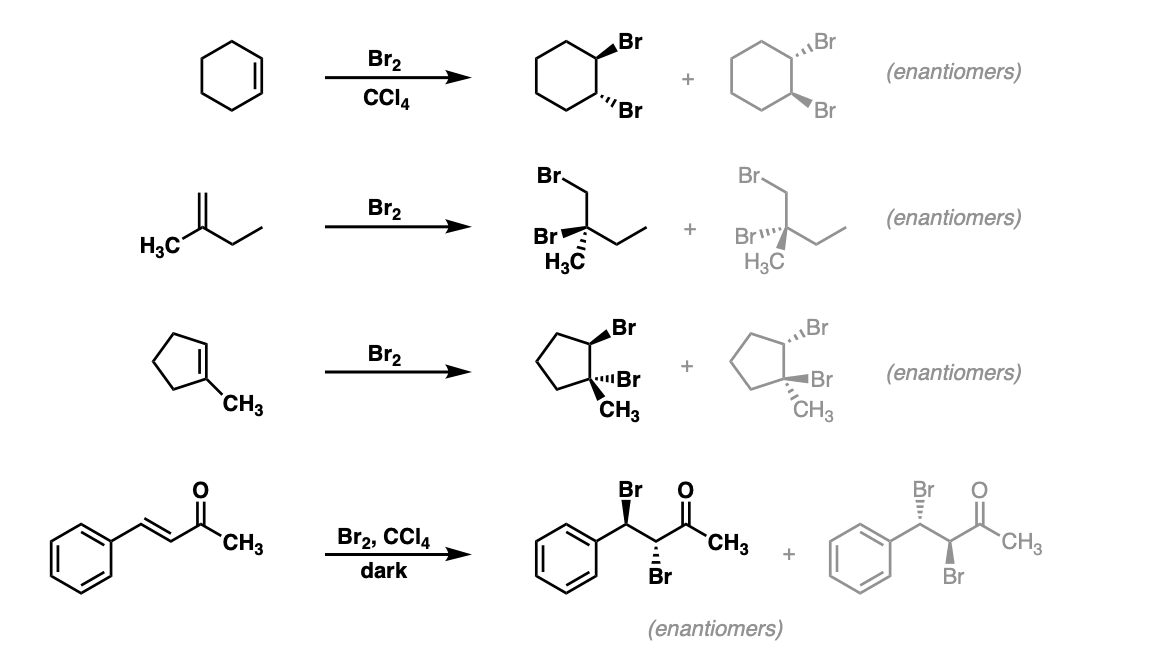
Bromination of alkenes with Br2 to give dibromides Master Organic Chemistry
For JEE (Main) and JEE(advanced)"Stereochemistry" - "Addition Of Bromine to Trans 2 Butene" (Lecture)For More Online Videos Click Here :http://www.youtube.co.

2butene molecule, illustration Stock Image F030/4729 Science Photo Library
Assertion : Cis -2-butene gives meso -2,3-butanediol with dilute alkaline KM nO4 solution. Reason: Dilute alkaline KM nO4 solution given trans addition with alkenes. View Solution. Q 2. Assertion :The addition of Br2 to 1-butene gives two optical isomers. Reason: The product contains one asymmetric carbon.

Addition of Br2 on cis 2 butene gives
Balanced Equation Mechanism. Reaction of (trans) 2-butene with Br 2 in H 2 O (solvent). Reaction of (cis) 2-butene with OsO 4 in H 2 O 2 (solvent). No Mechanism just draw the product (s). There are 2 steps to solve this one.
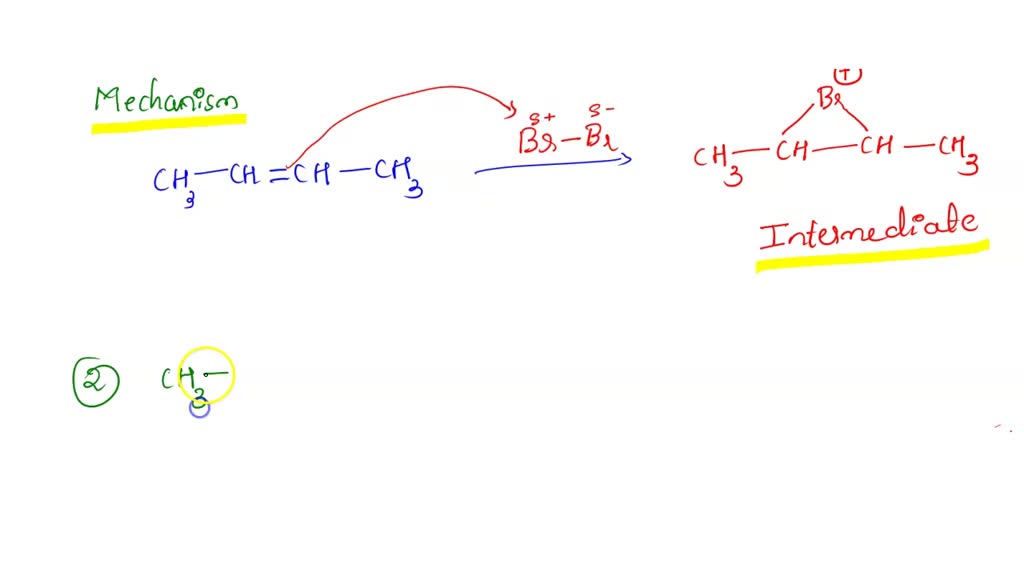
SOLVED Alkenes Draw the intemediate bromonium ion Part A Draw the intermediate that is common
Quantity Value Units Method Reference Comment; IE (evaluated) 9.10 ± 0.01: eV: N/A: N/A: L: Quantity Value Units Method Reference Comment; Proton affinity (review) 747.

2Butene, 2bromo SIELC Technologies
Notice: Except where noted, spectra from this collection were measured on dispersive instruments, often in carefully selected solvents, and hence may differ in detail from measurements on FTIR instruments or in other chemical environments. More information on the manner in which spectra in this collection were collected can be found here. Notice: Concentration information is not available for.

Addition of `Br_(2)` to trans2butene would give a product which is Sarthaks eConnect
$\begingroup$ First $\ce{Br2}$ is forming first a positive ion with one $\ce{Br}$ atom fixed on the double bond. It makes a positively charged triangular structure with the two central carbon atoms and one bromine atom. Then a negative ion may react with this positive ion.

(Z) 2 Butene reacts with Br2/H2O the resulting bromohydrin when treated with methoxide in
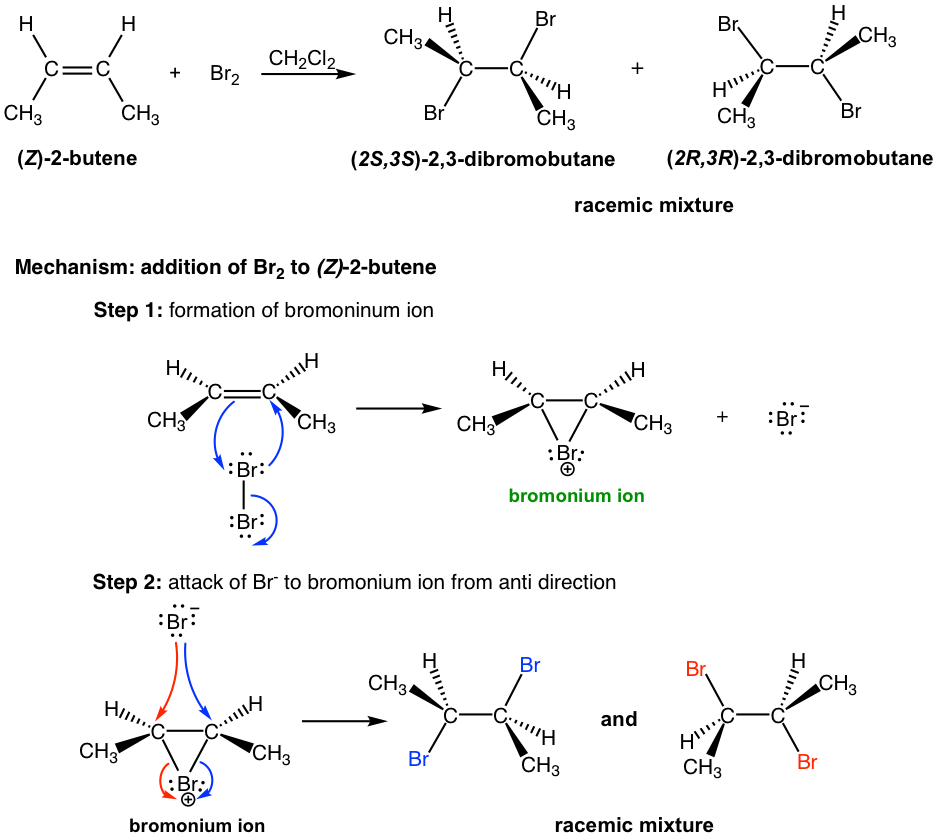
10.4 Reactions of Alkenes Addition of Bromine and Chlorine to Alkenes Chemistry LibreTexts
Trans-2-butene+Br2 CCl4 −−− →. View Solution. Q 4. Assertion :The addition of Br2 to 1-butene gives two optical isomers. Reason: The product contains one asymmetric carbon. View Solution. Q 5. Consider the reaction of trans- 2 -butene with Br2 in CH 2Cl2 . Which statement concerning this reaction is correct?

10.4 Reactions of Alkenes Addition of Bromine and Chlorine to Alkenes Chemistry LibreTexts
Stereochemistry of addition of Bromine to Cis and Trans-2-Butene. Bromine adds on to the cis isomer giving rise to (±)-2,3-Dibromobutane while the trans isomer leads to meso-Dibromobutane exclusively. These results are consistent with the two step mechanism with bromonium ion intermediate. The carbocation intermediate would have given rise to.
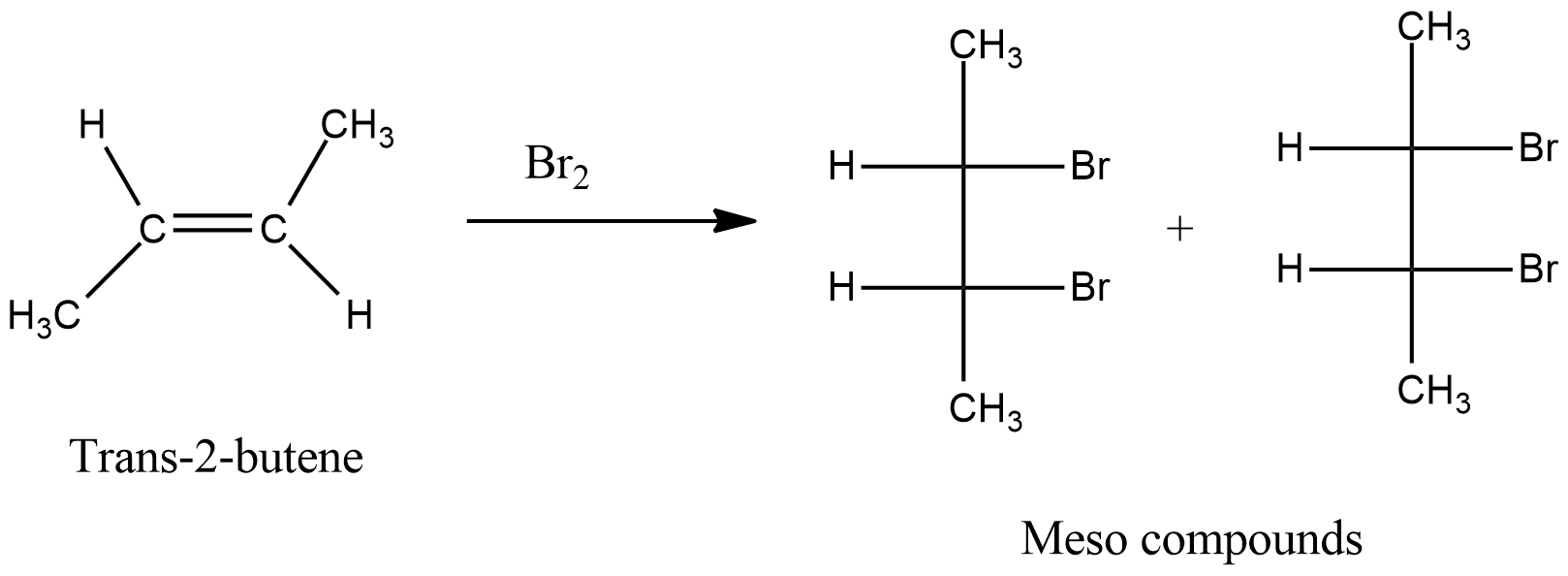
When trans2butene is reacted with B{{r}_{2}} then product is formedA. Racemic2,3
Data from NIST Standard Reference Database 69: NIST Chemistry WebBook The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment.
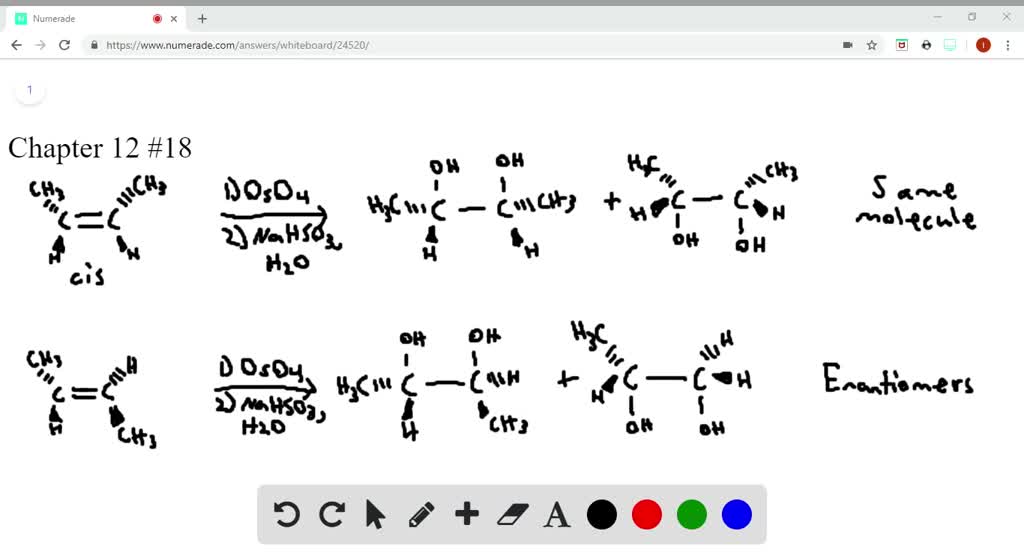
Reaction of (trans) 2butene with Brzin HzO(solvent)_… SolvedLib
What is the structure and formula of trans-2-butene? Learn more about this organic compound and its properties with this interactive molecular model from Purdue University.