
1Chlorobutane 99 500ml APC Pure
The bacterium Rhodococcus rhodochrous NCIMB 13064, isolated from an industrial site, could use a wide range of 1-haloalkanes as sole carbon source but apparently utilized several different mechanisms simultaneously for assimilation of substrate. Catabolism of 1-chlorobutane occurred mainly by attack.

1Chlorobutane, Merck
Other names: n-Butyl chloride; n-Propylcarbinyl chloride; Butyl chloride; 1-Chlorobutane; n-C4H9Cl; Chlorure de butyle; NBC wormer; NCI-C06155; Sure Shot; NSC 8419 Permanent link for this species. Use this link for bookmarking this species for future reference. Information on this page: Notes; Other data available: Gas phase thermochemistry data

1Chlorobutane, 98, Spectrum Chemical Fisher Scientific
1-Chlorobutane. Molecular Formula CHCl. Average mass 92.567 Da. Monoisotopic mass 92.039276 Da. ChemSpider ID 7714.

1Chlorobutane, Merck
The continuous-flow syntheses of 1-chlorobutane and 1-bromobutane were achieved in the gas phase starting from 1-butanol, at 130-170 °C and atmospheric pressure.An aqueous mixture of 1-butanol and commercial hydrochloric or hydrobromic acids (37 and 48%, respectively) was fed into a plug-flow catalytic reactor loaded with zinc chloride (ZnCl 2: 5 and 15 wt%) or a phosphonium salt (n-Bu 4 P.
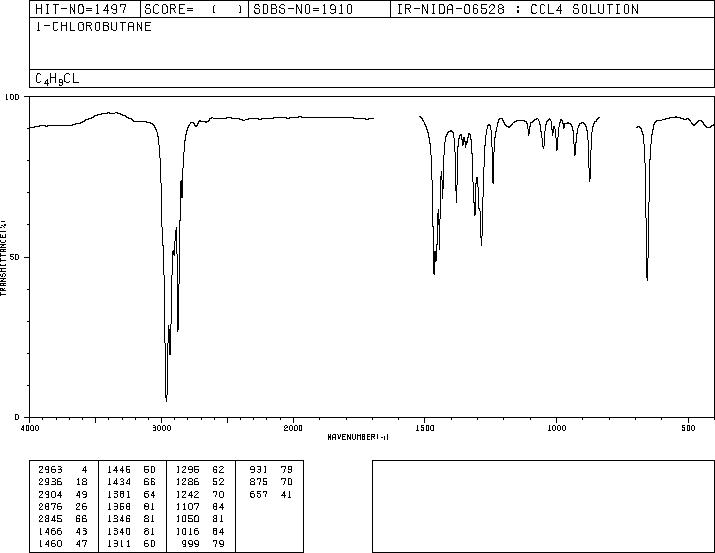
1Chlorobutane(109693) IR Spectrum
Aggregated GHS information provided by 38 companies from 1 notifications to the ECHA C&L Inventory. Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with.
1Chlorobutane, HPLC RCI LABSCAN LIMITED (EN)
1-Chlorobutane is an alkyl halide with the chemical formula CH 3 (CH 2) 3 Cl. It is a colorless, flammable liquid. Preparation and reactions. It can be prepared from 1-butanol by treatment with hydrogen chloride. It reacts with lithium metal to give n-butyllithium:
1Chlorobutane >99.0(GC) 25mL
CAS Registry Number: 927-73-1; Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. Other names: 1-Butene, 4-chloro- Permanent link for this species. Use this link for bookmarking this species for future reference. Information on this page:
1Chlorobutane2,3dione SIELC Technologies
Safety Data Sheet for 1-Chlorobutane 801640. Material Safety Data Sheet or SDS for 1-Chlorobutane 801640 from Merck for download or viewing in the browser. Catalog Number 801640. Product Name 1-Chlorobutane. Select Language.

1CHLOROBUTANE 100ml
Synthesis Reference(s): Journal of the American Chemical Society, 87, p. 2500, 1965 DOI: 10.1021/ja01089a041 The Journal of Organic Chemistry, 59, p. 4687, 1994.

1Chlorobutane CAS 109693 SDCY CHEM
Based on a Henry's Law constant of 0.0167 atm-cu m/mol(1), the volatilization of n-butyl chloride from a model river 1 m deep, flowing at 1 m/sec with a 3 m/sec wind is 2.9 hr(2). The volatilization half-life from a model pond is 34 hr(3). Due to its high vapor pressure and Henry's Law constant and low adsorptivity to soil, n-butyl chloride.

1CHLOROBUTANE Crescendo
1-Chlorobutane. CH3(CH2)3Cl. Synonyms: Butyl chloride, 1-Chlorobutane. CAS 109-69-3. Molecular Weight 92.57. Browse 1-Chlorobutane and related products at Merck.
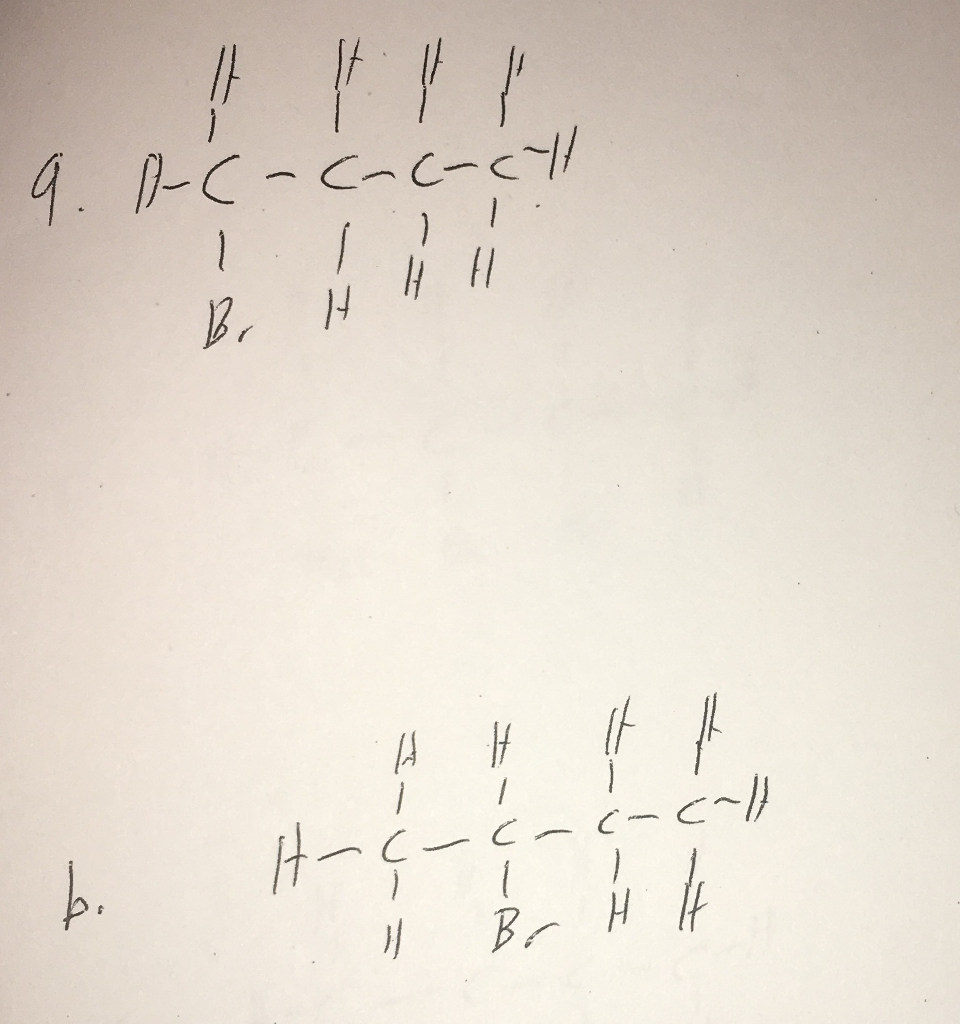
Solved Starting with 1chlorobutane show how each of the
The 2D chemical structure image of 1-Chlorobutane is also called skeletal formula, which is the standard notation for organic molecules. The carbon atoms in the chemical structure of 1-Chlorobutane are implied to be located at the corner(s) and hydrogen atoms attached to carbon atoms are not indicated - each carbon atom is considered to be associated with enough hydrogen atoms to provide the.

1chlorobutane, 99+ , pur, Thermo Scientific Chemicals Fisher Scientific
Upper/lower flammability or explosive limits Upper explosion limit: 10,1 %(V) Lower explosion limit: 1,8 %(V) Vapour pressure 120,6 hPa at 20 °C - OECD Test Guideline 104; Vapour density 3,2 - (Air = 1.0) Relative density 0,886 g/cm3 at 25 °C - lit. 0,88 at 20 °C; Water solubility ca.0,11 g/l at 20 °C - OECD Test Guideline 105- partly soluble

109693・1クロロブタン・1Chlorobutane・02303593・02703596【詳細情報】|試薬富士フイルム和光純薬
1-Chlorobutane is an isomer of chlorobutane. [1] It undergoes stoichiometric and catalytic dehydrochlorination on reacting with nanocrystalline MgO (magnesium oxide) to generate isomers of butene. [2] Viscosities of the binary mixtures of pentyl acetate and 1-chlorobutane and 1-chlorobutane and acetonitrile has been studied.

Chlorobutane, molecular model Stock Image C029/0930 Science Photo Library
1-Chlorobutane is a highly flammable, clear, colorless liquid at standard temperature and pressure. The density is 0.886 g/cm3, which is lower than that of water. It does not react with water, is classified as highly flammable, but it is neither an oxidizer nor an explosive. However, vapors can form explosive mixtures with air.
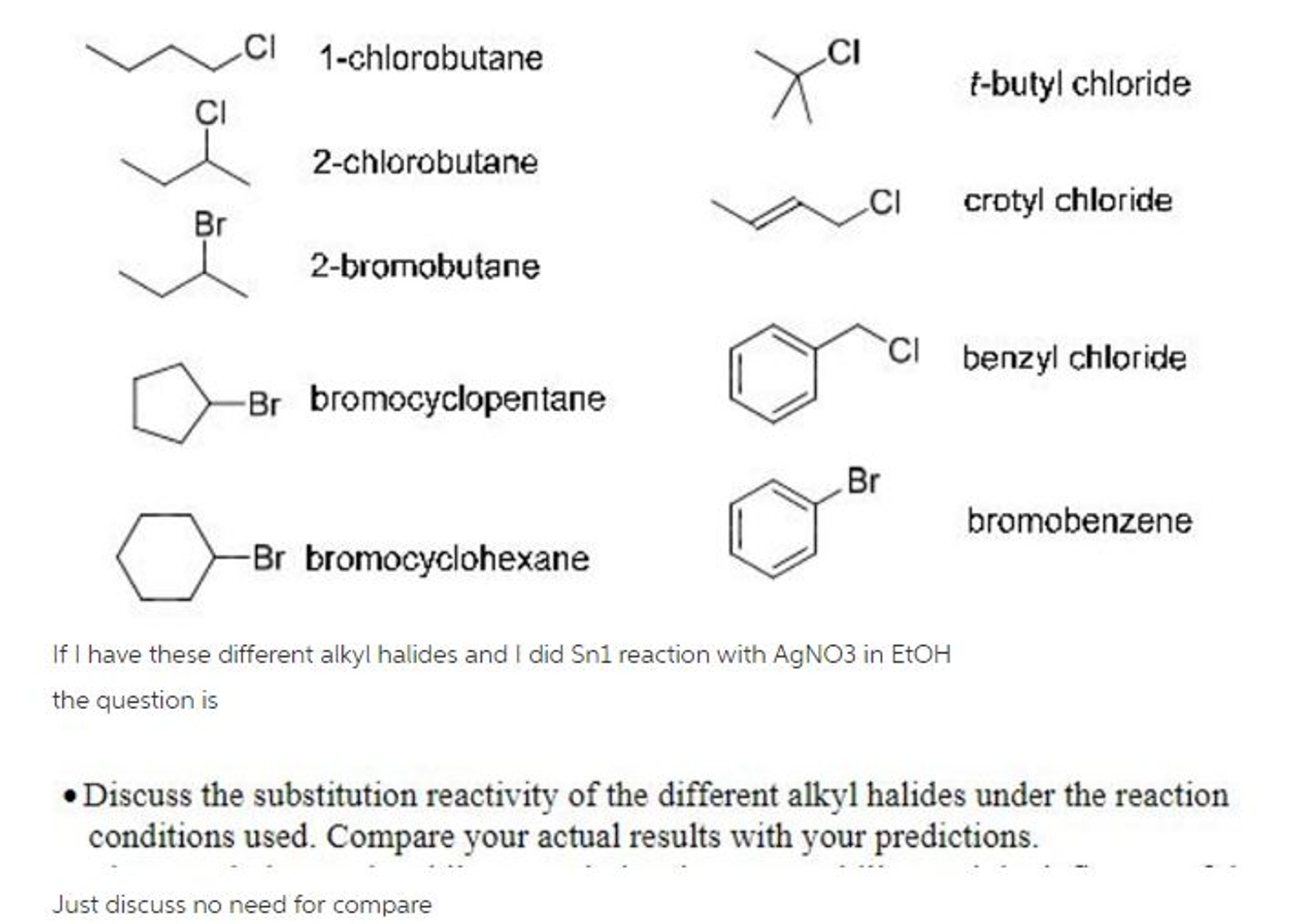
Solved Cl 1chlorobutane Cl tbutyl chloride Cl
synthesis of 1-butanol from 2-chlorobutane. Here's the best way to solve it. 100% (2 ratings) Share Share. View the full answer.